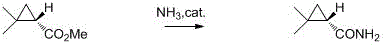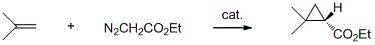A kind of preparation method of chiral dimethyl cyclopropanamide
A technology of dimethylcyclopropanamide and dimethylcyclopropanamide, which is applied in the preparation of chiral dimethylcyclopropanamide compounds, in the field of dimethylcyclopropanamide, can solve the problem of the disadvantages of the preparation method low rate and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
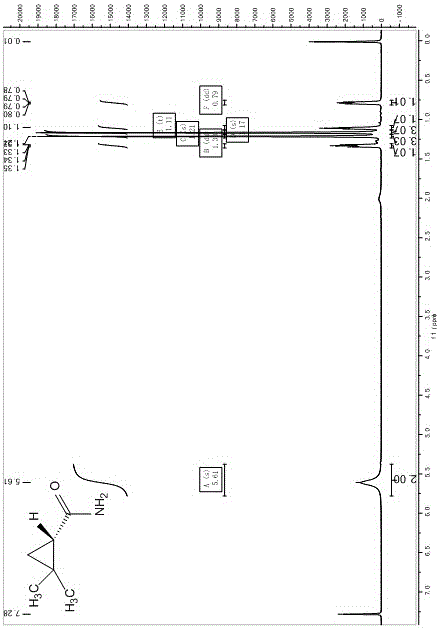
[0023]
[0024] The preparation method of above-mentioned a kind of (S)-ethyl dimethylcyclopropanecarboxylate specifically comprises the steps:
[0025] In a 1000 mL three-necked flask equipped with a thermometer and a dropping funnel, add (R,R)-(+)-2,2'-isopropylidenebis(4-tert-butyl-2-oxazoline) ( 1.74 g, 5.94 mmol), cuprous trifluoromethanesulfonate (1.14 g, 5.4 mmol) and dichloromethane (250 mL) were stirred at room temperature (25~30 ℃) for 2 h. Cool the reaction system to -5~0°C, add isobutylene to saturation, then slowly add ethyl diazoacetate (114 g, 1 mol) dropwise, control the temperature of the reaction system at 0°C, and slowly warm up to room temperature (25 ℃), continue to stir and react for 1 h; after TLC detects that the EDA reaction is complete, the system is concentrated under reduced pressure (T 98 %, e.e. value >95%.
Embodiment 2
[0027]
[0028] Above-mentioned a kind of (S)-dimethyl cyclopropanecarboxylate preparation method specifically comprises the steps:
[0029] In a 1000 mL three-necked flask equipped with a thermometer and a dropping funnel, add (R,R)-(+)-2,2'-isopropylidenebis(4-tert-butyl-2-oxazoline) ( 1.74 g, 5.94 mmol), cuprous trifluoromethanesulfonate (1.14 g, 5.4 mmol) and dichloromethane (250 mL) were stirred at room temperature (25~30 ℃) for 2 h. Cool the reaction system to -5~0°C, add isobutylene to saturation, then slowly add isopropyl diazoacetate (129 g, 1 mol) dropwise, control the temperature of the reaction system at 0°C, and slowly warm up to room temperature after the addition ( 25 ℃), continue to stir the reaction for 1 h; after the completion of the EDA reaction by TLC, the system is concentrated under reduced pressure (T 98 %, e.e. Value >95%.
Embodiment 3
[0031]
[0032] The preparation method of above-mentioned a kind of (S)-dimethylcyclopropanecarboxylate, specifically comprises the steps:
[0033] In a 1000 mL three-necked flask equipped with a thermometer and a dropping funnel, add (R,R)-(+)-2,2'-isopropylidenebis(4-tert-butyl-2-oxazoline) ( 1.74 g, 5.94 mmol), cuprous trifluoromethanesulfonate (1.14 g, 5.4 mmol) and dichloromethane (250 mL) were stirred at room temperature (25~30 ℃) for 2 h. Cool the reaction system to -5~0°C, add isobutylene to saturation, then slowly add the above-prepared methyl diazoacetate (101 g, 1 mol) dropwise, control the temperature of the reaction system to 0°C, and slowly heat up the system after the addition After reaching room temperature (25 °C), continue to stir and react for 1 h; after the completion of the EDA reaction by TLC, the system is concentrated under reduced pressure (T 98 %, e.e. values >95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com