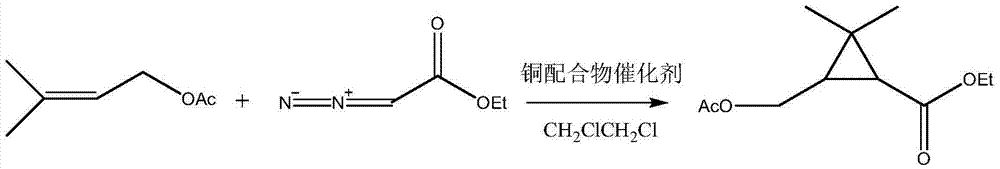
3-acetoxy methyl-ethyl-2,2-dimethylcyclopropanecarboxylate synthesis method
A technology of ethyl cyclopropanyl formate and acetoxymethyl, which is applied in the field of synthesis of 3-acetoxymethyl-2,2-dimethyl-cyclopropanyl ethyl formate, can solve the problem of stirring reaction Long time, unstable product quality, complicated operation steps and other problems, to achieve the effect of shortened reaction time, stable product quality and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
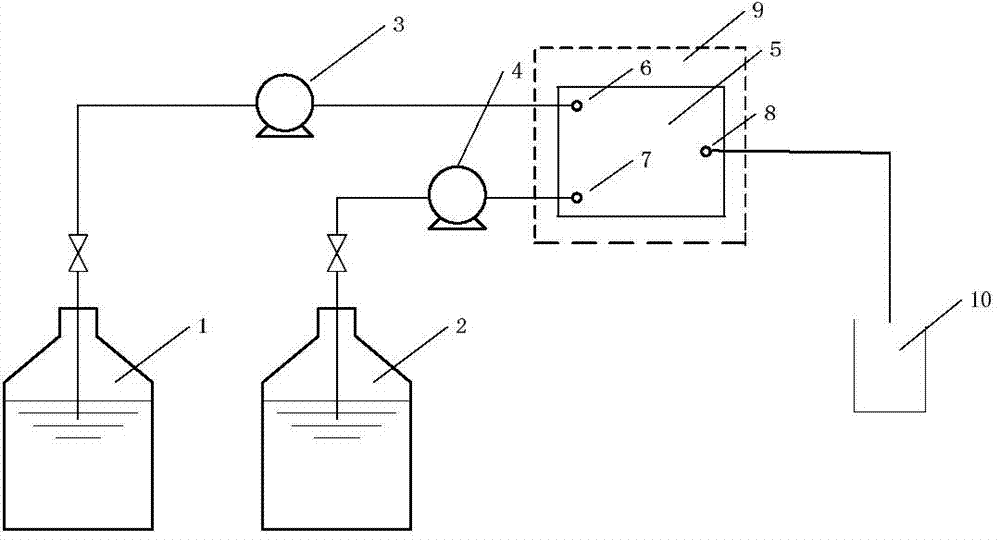
[0020] Use constant current pump 3 (Series II digital pump, Chrom Tech, Inc.) to control ethyl diazonium acetate solution 1, and use constant current pump 4 to control the output of prenyl acetate solution 2, so that the prenyl acetate solution and the The ethyl diazoacetate solution is simultaneously introduced into the inlet of the ethyl diazoacetate solution and the inlet of the prenyl acetate in the microreactor, and contacts in the microreactor to initiate the cyclopropanation reaction.
[0021] Microreactor:
[0022] CNC precision machining preparation
[0023] Material 316L stainless steel
[0024] Hydraulic diameter 0.4mm
[0025] Effective volume 230μl
[0026] Process conditions:
[0027] Ethyl diazoacetate solution (ethyl diazoacetate 71wt.%, 1,2-dichloroethane 29wt.%)
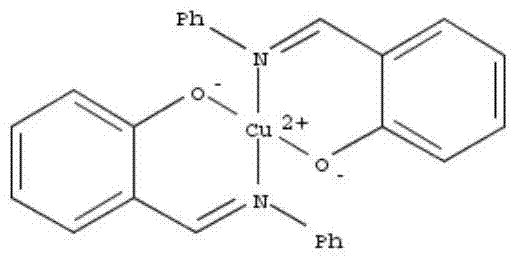
[0028] Prenyl acetate solution (prenyl acetate 97wt.%, copper complex catalyst 3wt.%)
[0029] Microreactor temperature control 110℃
[0030] reaction pressure
[0031] Prenyl acetate: ethyl...
Embodiment 2
[0039] According to the same method as in Example 1, the temperature of the microreactor was controlled to 100°C, and other experimental conditions and analysis methods were the same as those in Example 1. Implementation results: 3-acetoxymethyl-2,2-dimethyl-cyclopropane ethyl formate 69%, raw material ethyl diazoacetate 31%.
Embodiment 3
[0041] According to the same method as in Example 1, the temperature of the microreactor was controlled to 90°C, and other experimental conditions and analysis methods were the same as those in Example 1. Implementation results: 32% ethyl 3-acetoxymethyl-2,2-dimethyl-cyclopropanecarboxylate, 68% ethyl diazoacetate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com