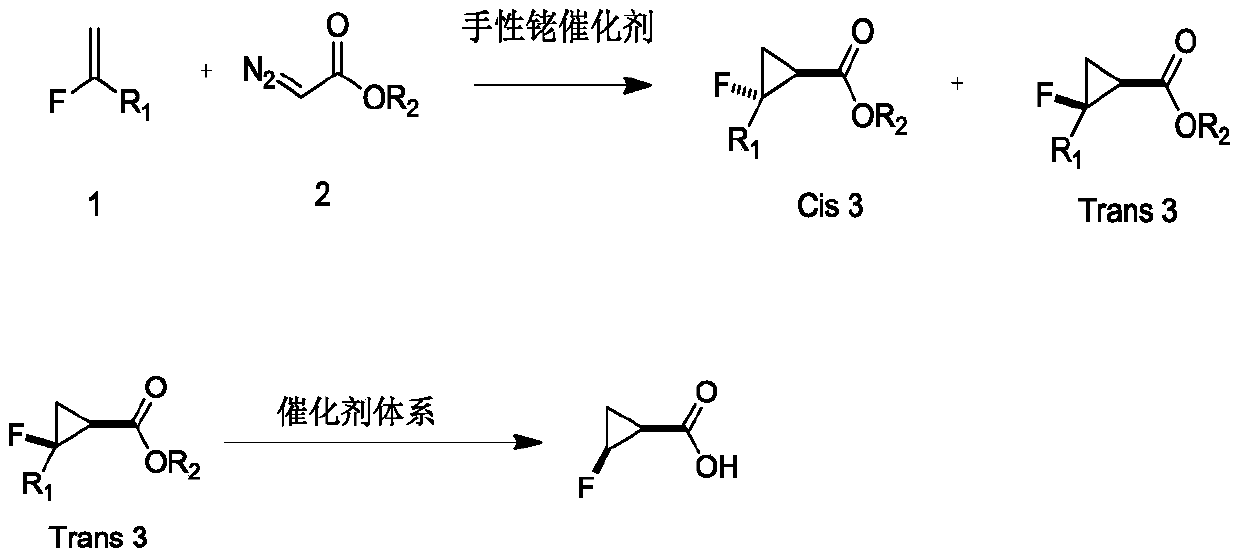
Novel method for asymmetric synthesis of (1S,2S)-2-fluorocyclopropanecarboxylic acid under catalysis of chiral rhodium catalyst
A technology of rhodium catalyst and fluorocyclopropane, which is applied in the field of asymmetric synthesis of 2-fluorocyclopropanecarboxylic acid catalyzed by chiral rhodium catalyst, which can solve the problems of high Trans/Cis ratio and no absolute configuration stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
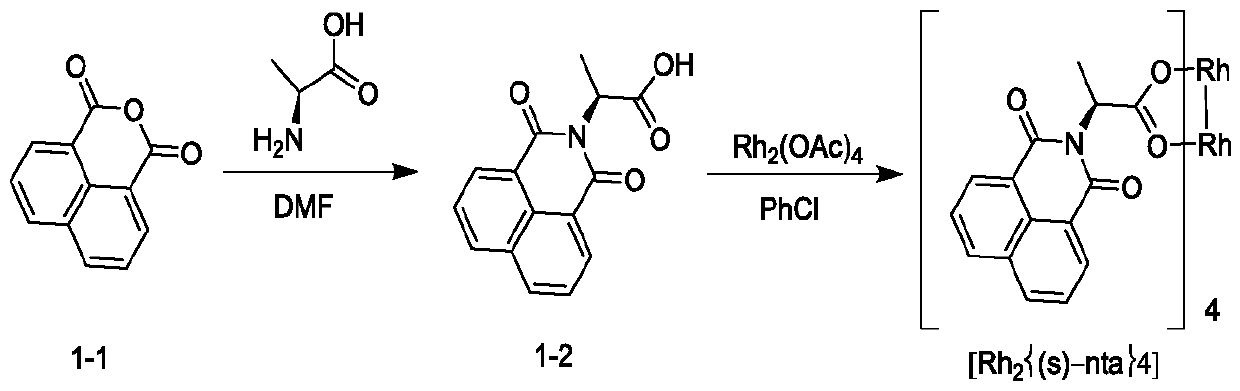
[0093] Example 1: Rh 2 {(s)-nta} 4 Synthesis
[0094]
[0095] Take a 500mL three-neck flask, add DMF (300mL), 1,8-naphthalene dicarboxylic anhydride (30 g, 151.4mmol), L-alanine (13.5g, 151.4mmol) in turn under stirring; heat up to 145°C-150°C The reaction was continued for 5 hours; the reaction solution was concentrated to obtain the crude compound 1-2; the crude product was purified by silica gel column to obtain 26.5 g of compound 1-2.
[0096] Take a 250mL three-neck flask, add chlorobenzene (150mL), compound 1-2 (12.2g, 45.2mmol) and dimeric rhodium acetate (5g, 11.3mmol) sequentially under stirring; heat up to 130°C-135°C, and reflux for 3 hours ; The there-necked flask is equipped with a vacuum distillation device, and the solvent is distilled while stirring the reaction until it does not drop; add n-hexane (60mL), make a slurry, filter, and dry the solid to obtain 14g Rh 2 {(s)-nta} 4 .
[0097] Compound 1-2, 1 HNMR (DMSO-d 6 ,400MHz):δ12.3(br,1H),8.49~8.53(...
Embodiment 2
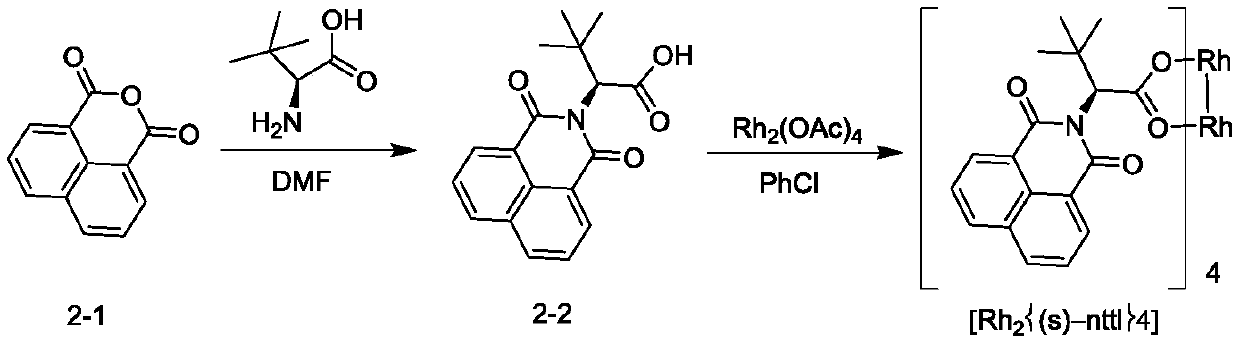
[0098] Example 2: Rh 2 {(s)-nttl} 4 Synthesis
[0099]
[0100] Take a 500mL three-neck flask, add DMF (300mL), 1,8-naphthalene dicarboxylic anhydride (30 g, 151.4mmol), L-tert-leucine (19.9g, 151.4mmol) in turn under stirring; The reaction was continued at °C for 5 hours; the reaction solution was concentrated to obtain a crude compound 2-2; the crude product was purified by a silica gel column to obtain 28 g of compound 2-2.
[0101] Take a 250mL three-neck flask, add chlorobenzene (150mL), compound 2-2 (14.1g, 45.2mmol) and dimerized rhodium acetate (5g, 11.3mmol) in sequence under stirring; heat up to 130°C-135°C, and reflux for 3 hours ; The vacuum distillation device is configured on the three-necked flask, and the solvent is distilled while stirring the reaction until it does not drop; Add n-hexane (60mL), make a slurry, filter, and dry the solid to obtain 16g Rh 2 {(s)-nttl} 4 .
[0102] Compound 2-2, 1 HNMR (CDCl 3 ,400MHz):δ12.1(br,1H),8.58~8.62(m,2H), 8.20...
Embodiment 3
[0103] Example 3: Rh 2 {(s)-bpttl} 4 Synthesis
[0104]
[0105] Take a 500mL three-neck flask, add DMF (300mL), 2,3-naphthalene dianhydride (30g, 151.4mmol) and L-tert-leucine (19.9g, 151.4mmol) in turn under stirring; heat up to 145°C-150°C The reaction was continued for 5 hours; the reaction solution was concentrated to obtain a crude compound 3-2; the crude product was purified by a silica gel column to obtain 27 g of compound 3-2.
[0106] Take a 250mL three-neck flask, add chlorobenzene (150mL), compound 3-2 (14.1g, 45.2mmol) and dimeric rhodium acetate (5g, 11.3mmol) in sequence under stirring; heat up to 130°C-135°C, and reflux for 3 hours ; The there-necked flask is equipped with a vacuum distillation device, and the solvent is distilled while stirring the reaction, and distilled until it does not drop; add n-hexane (60mL), make a slurry, filter, and dry the solid to obtain 15g Rh 2 {(s)-bpttl} 4 .
[0107] Compound 3-2, 1 HNMR (CDCl 3 ,400MHz):δ12.7(br,1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com