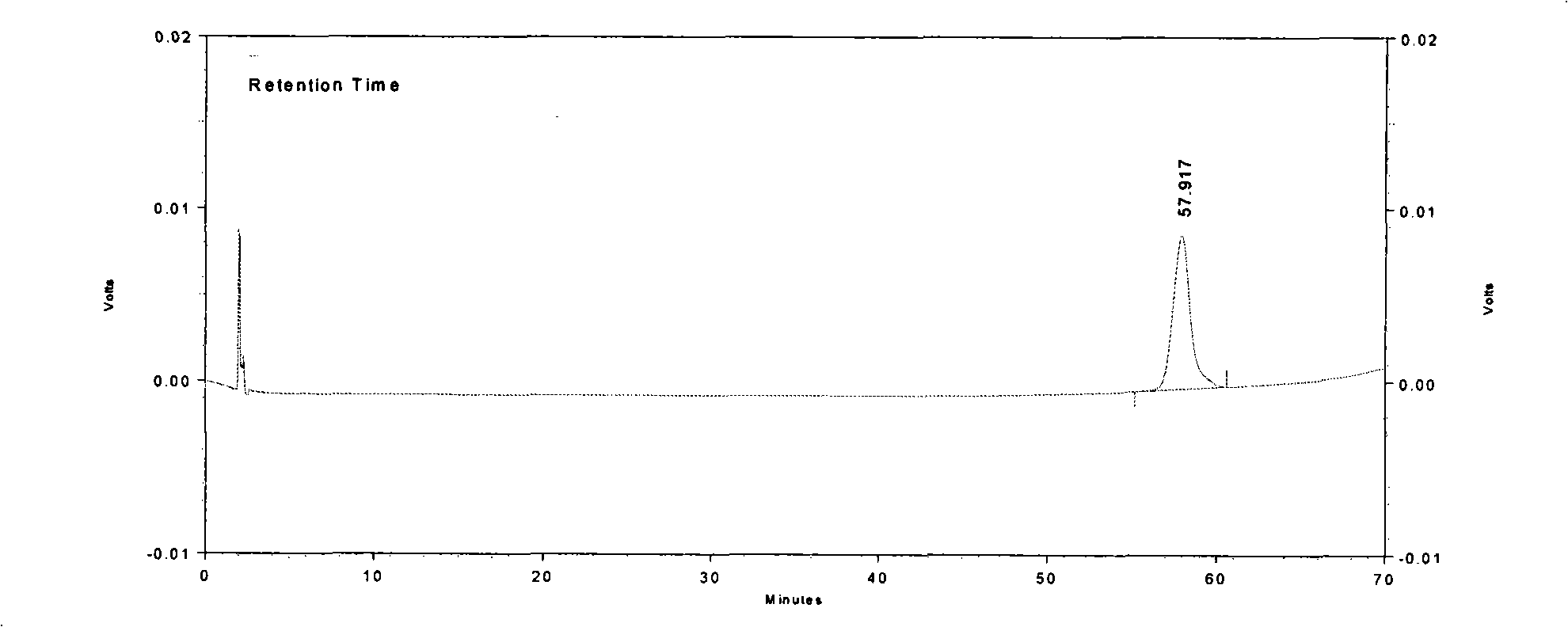
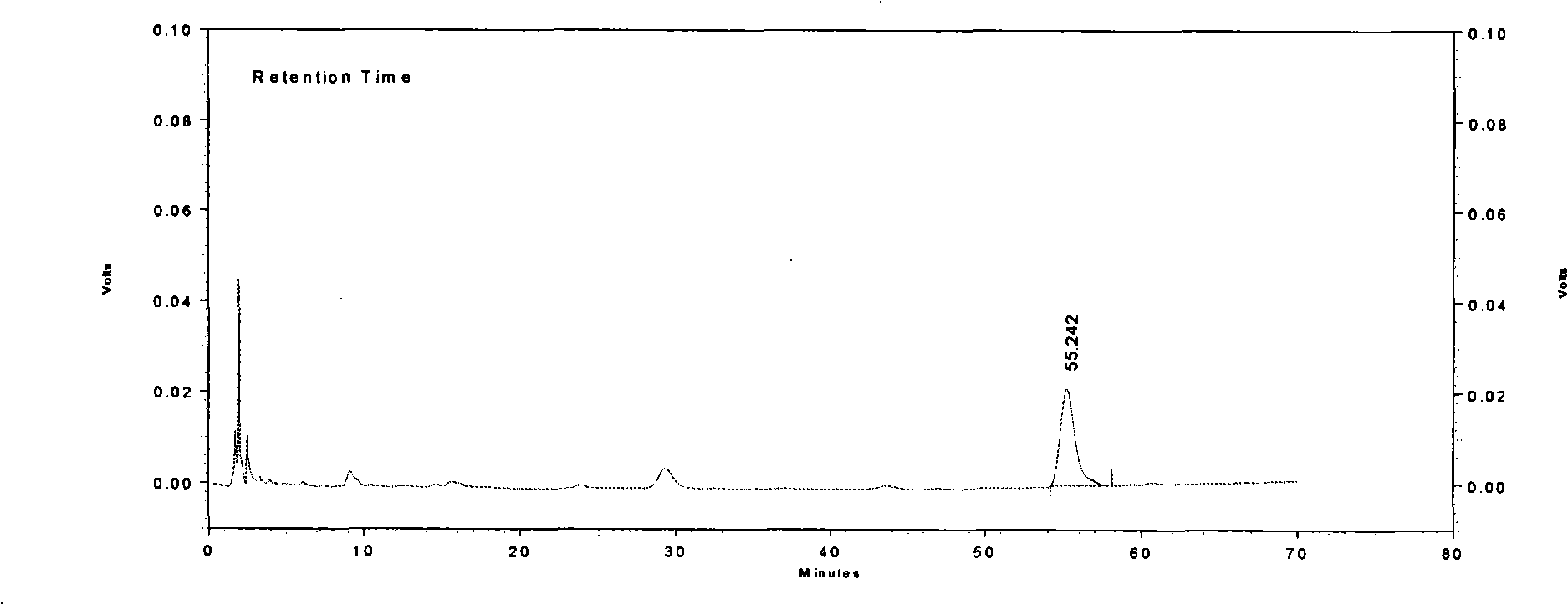
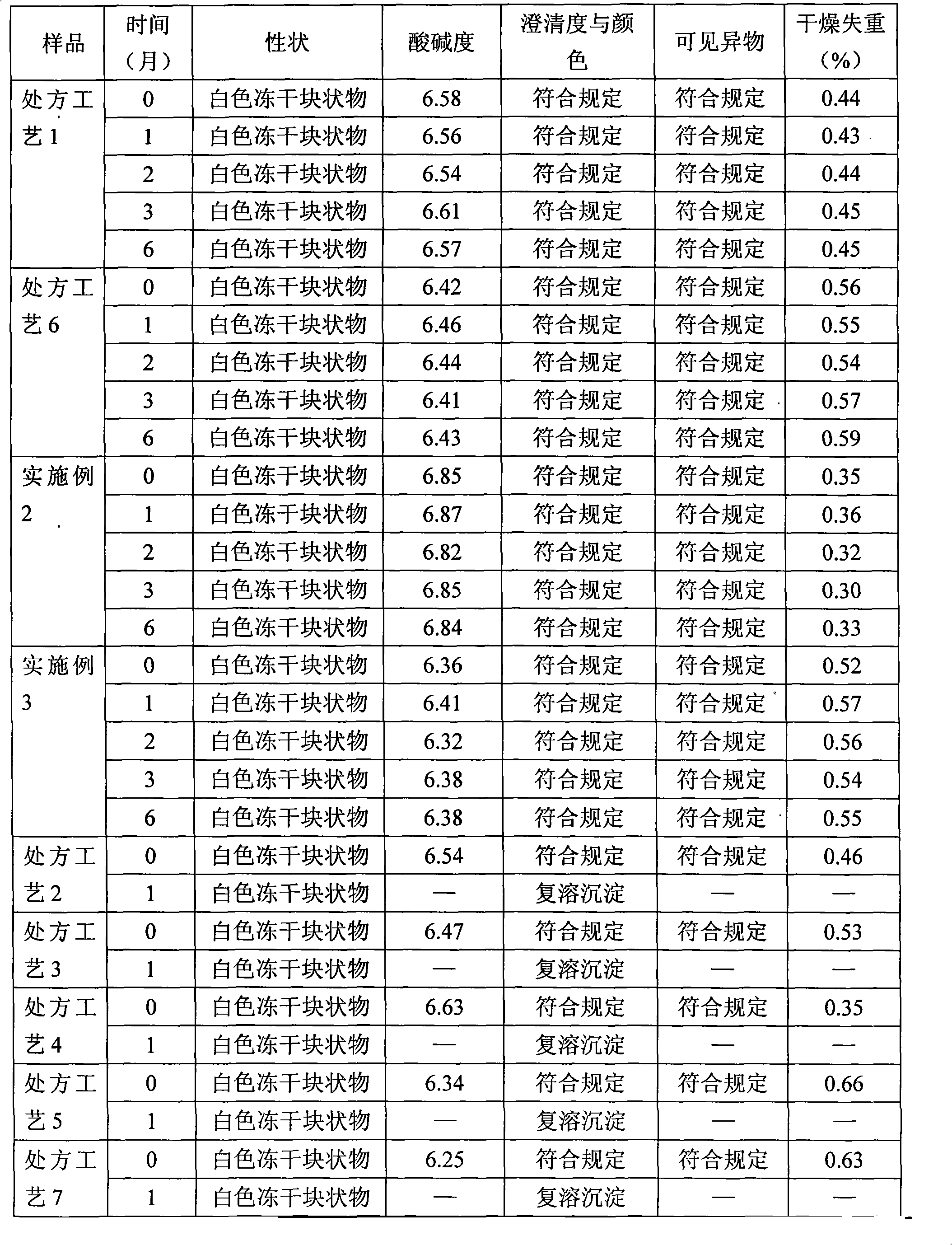
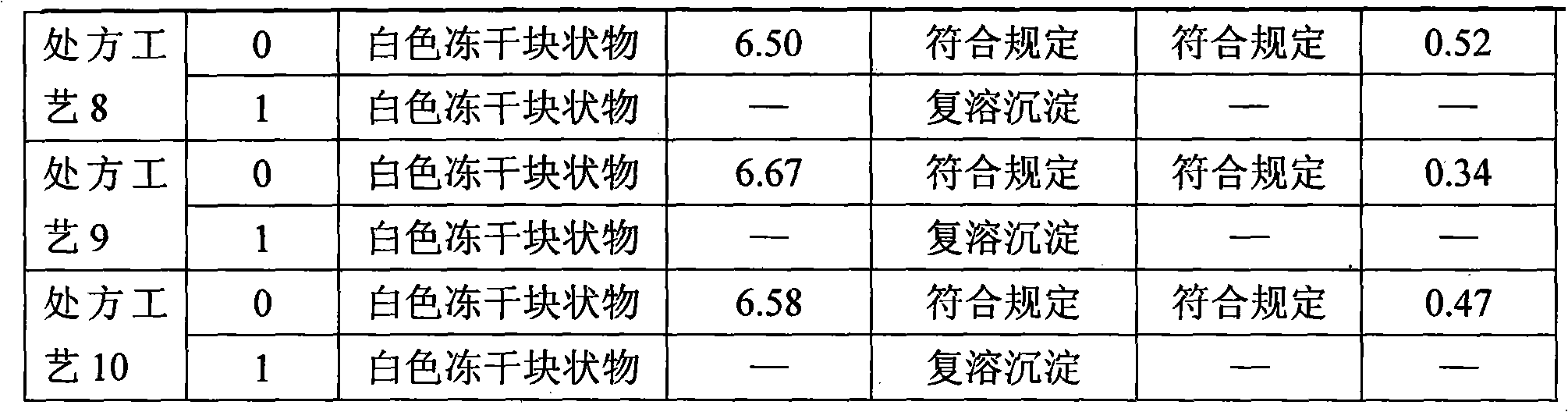
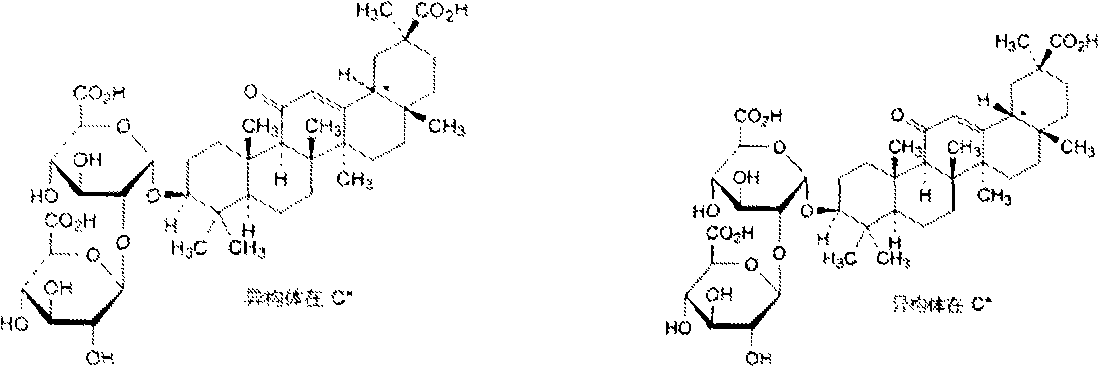
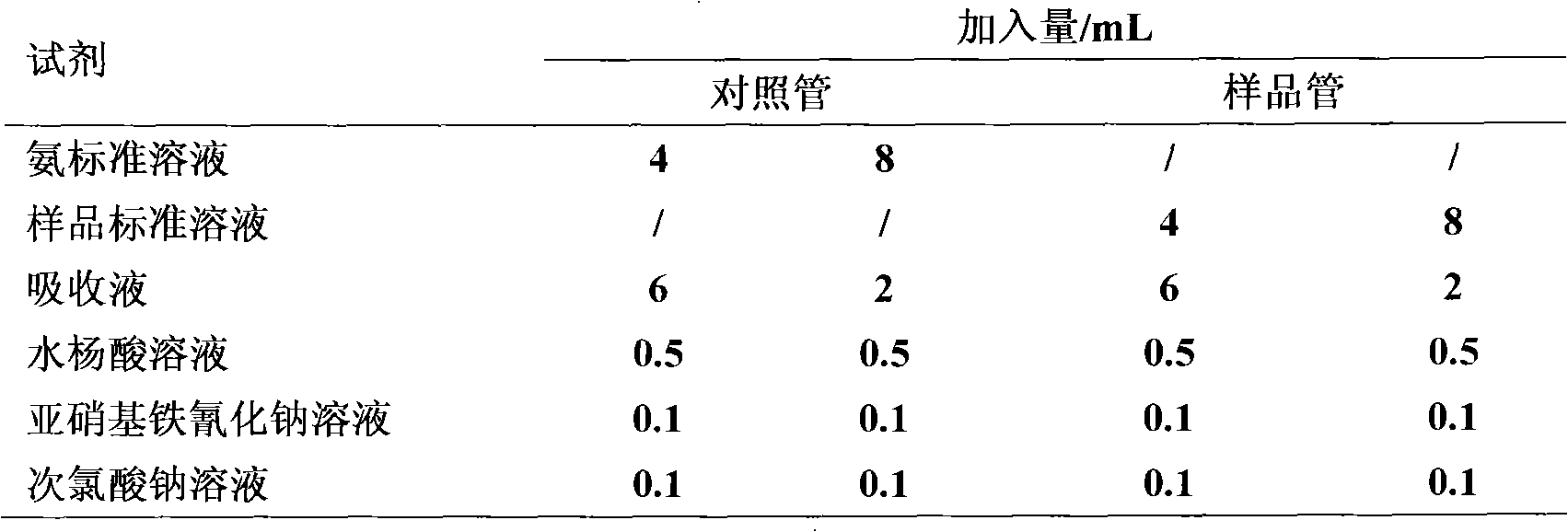
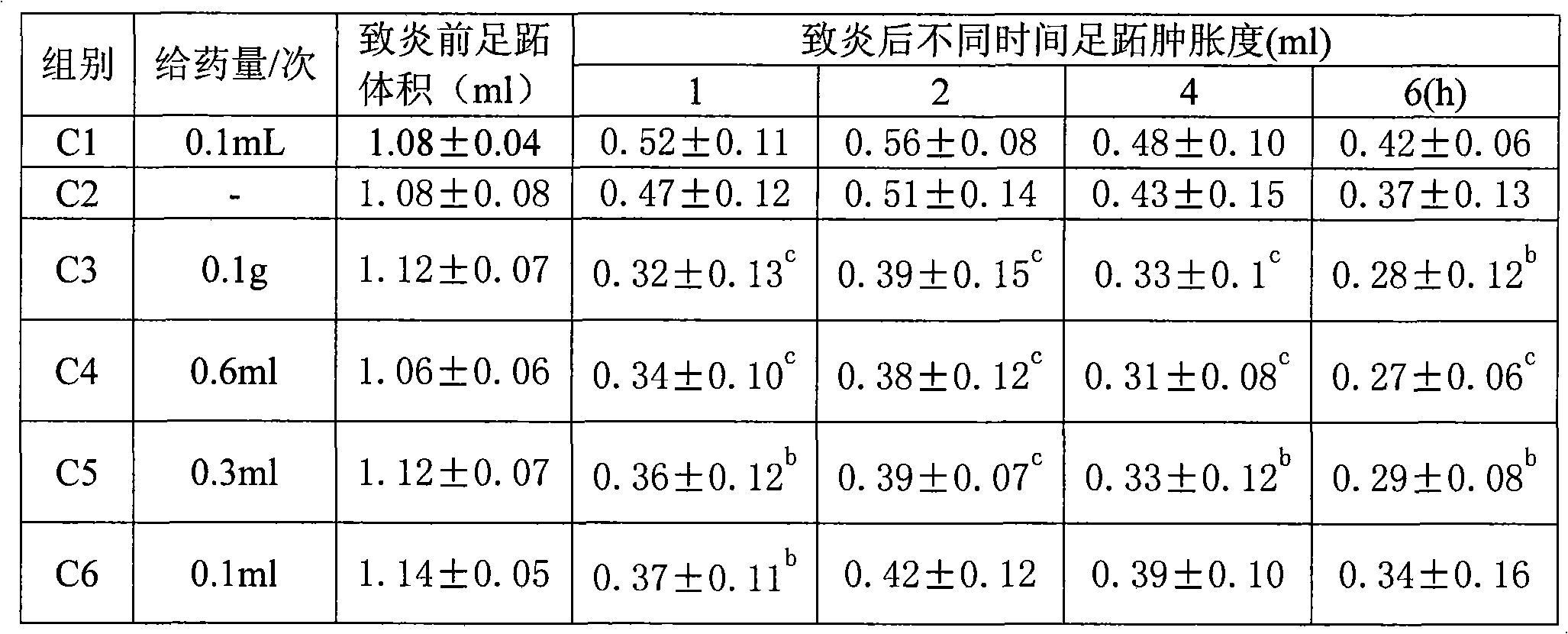
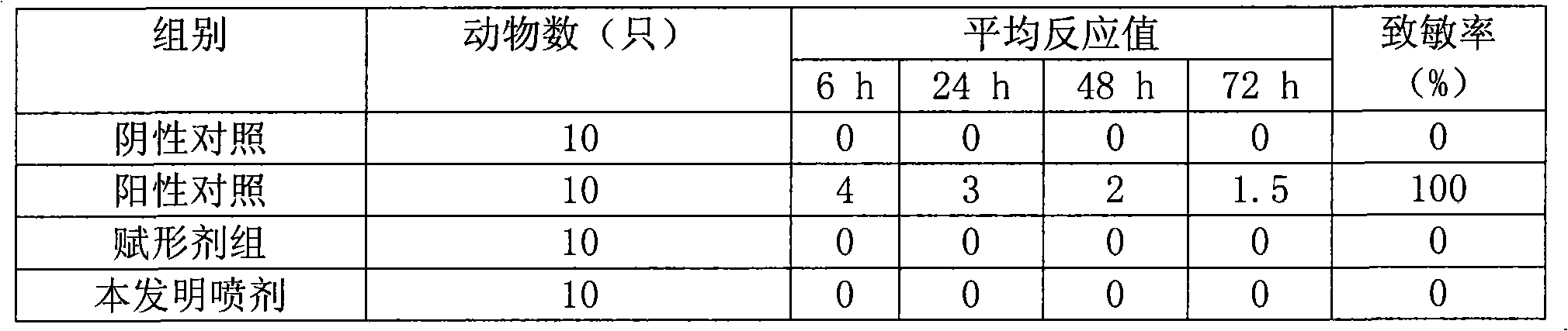
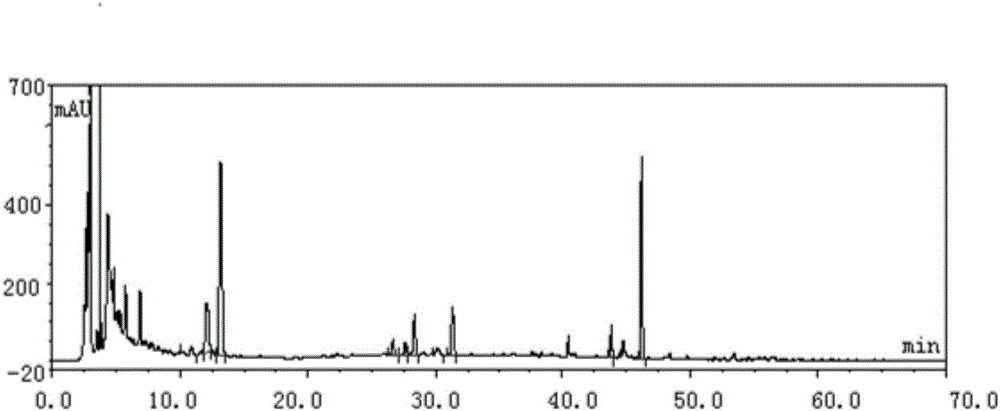
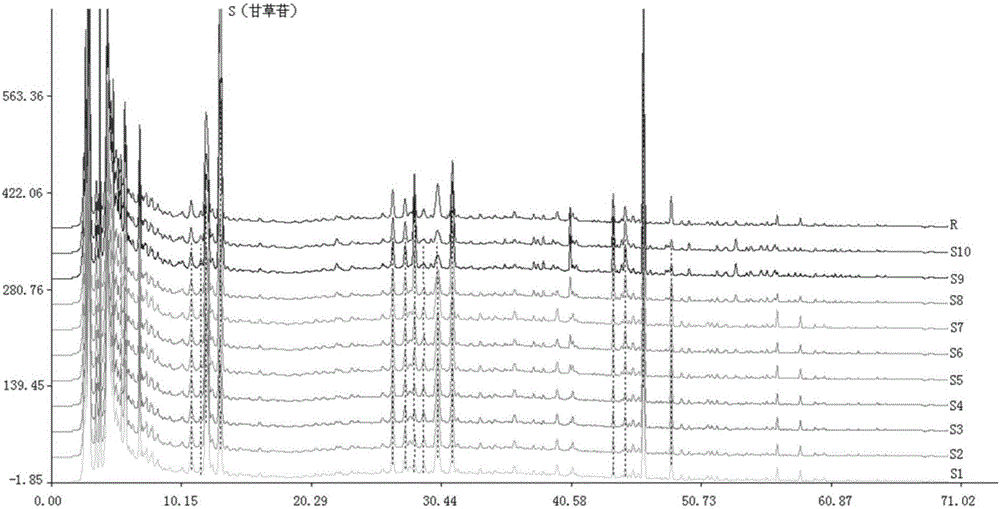
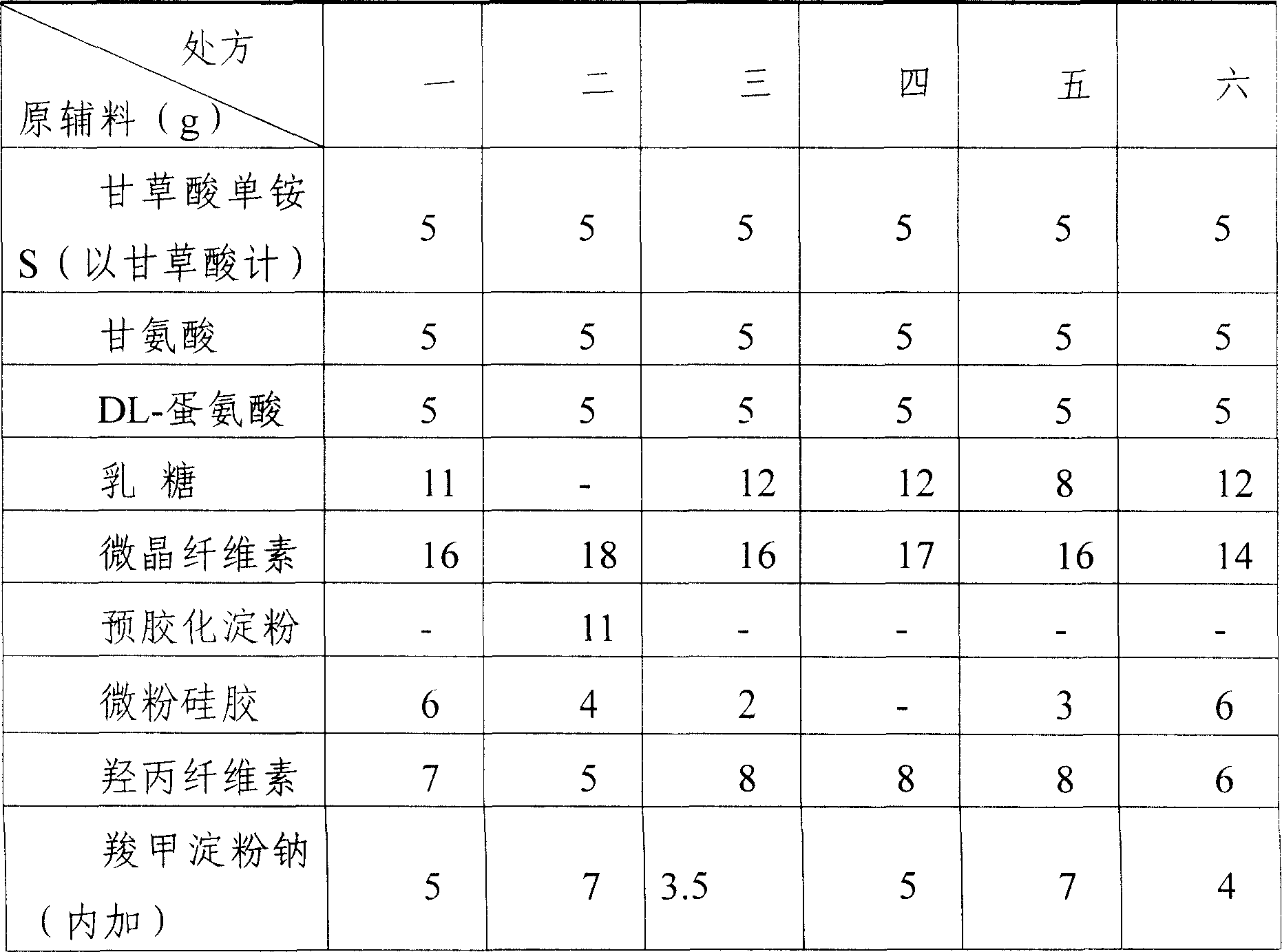
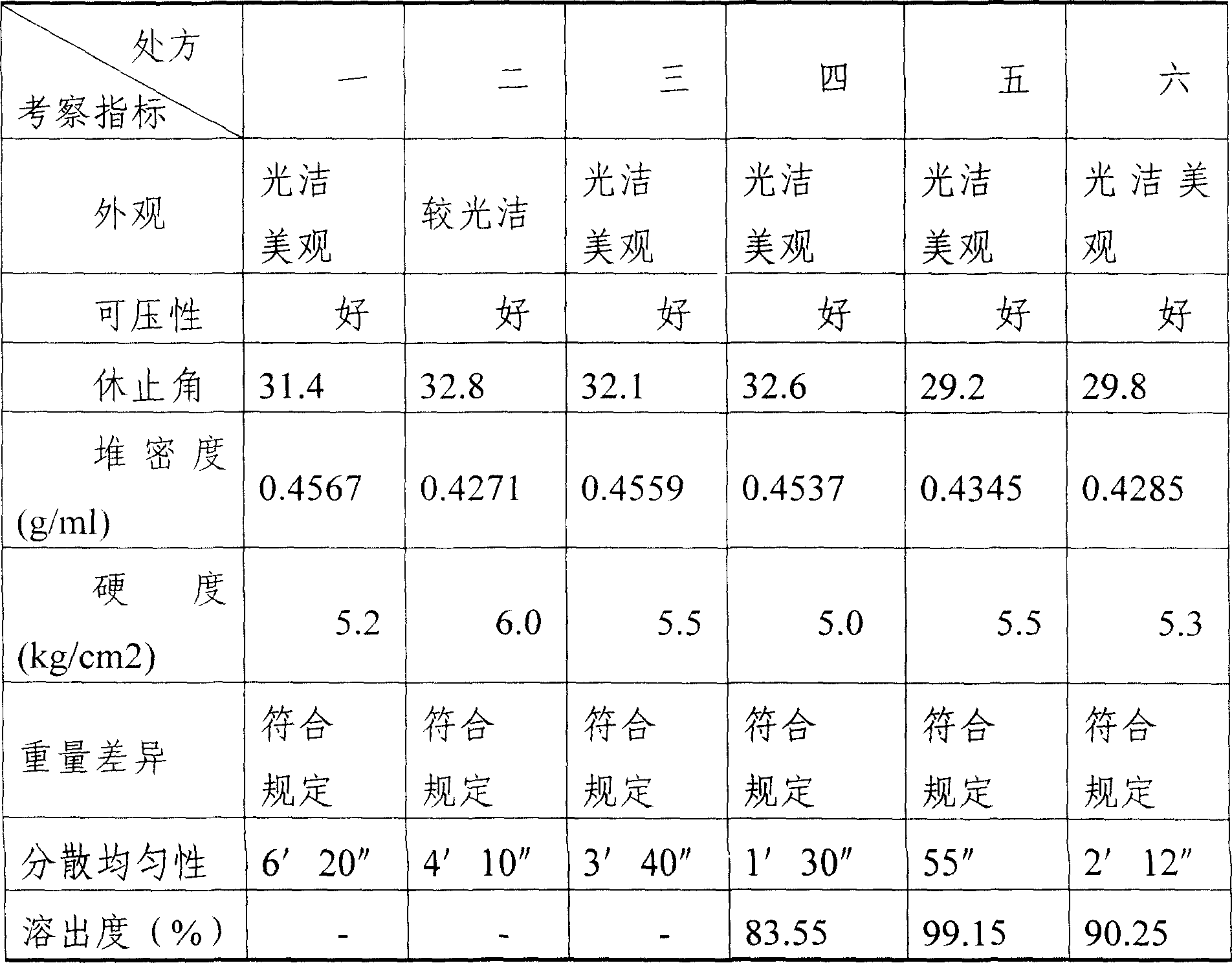
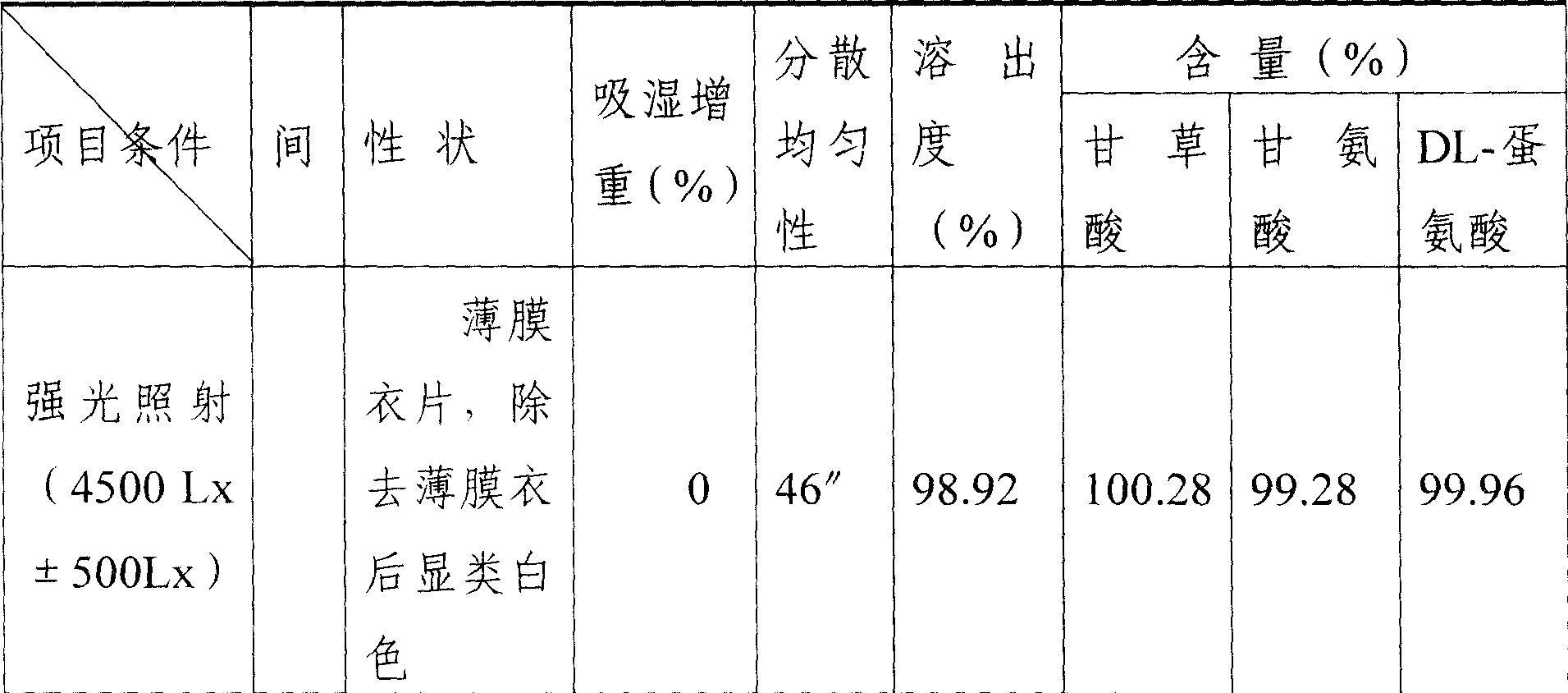
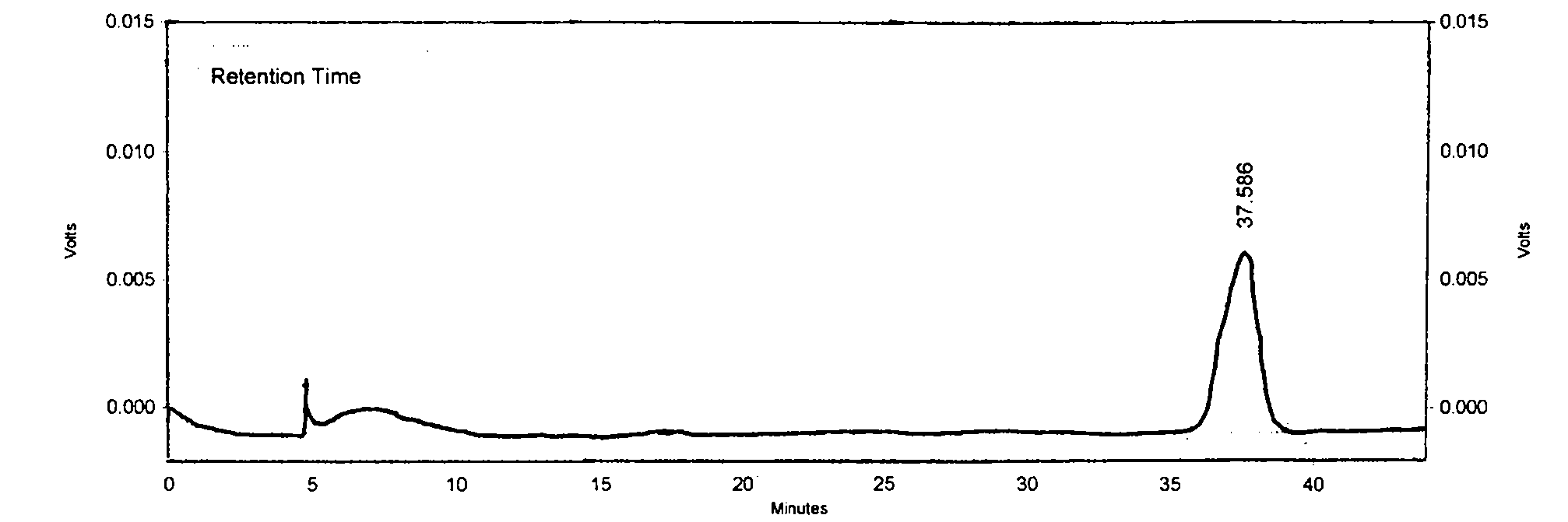
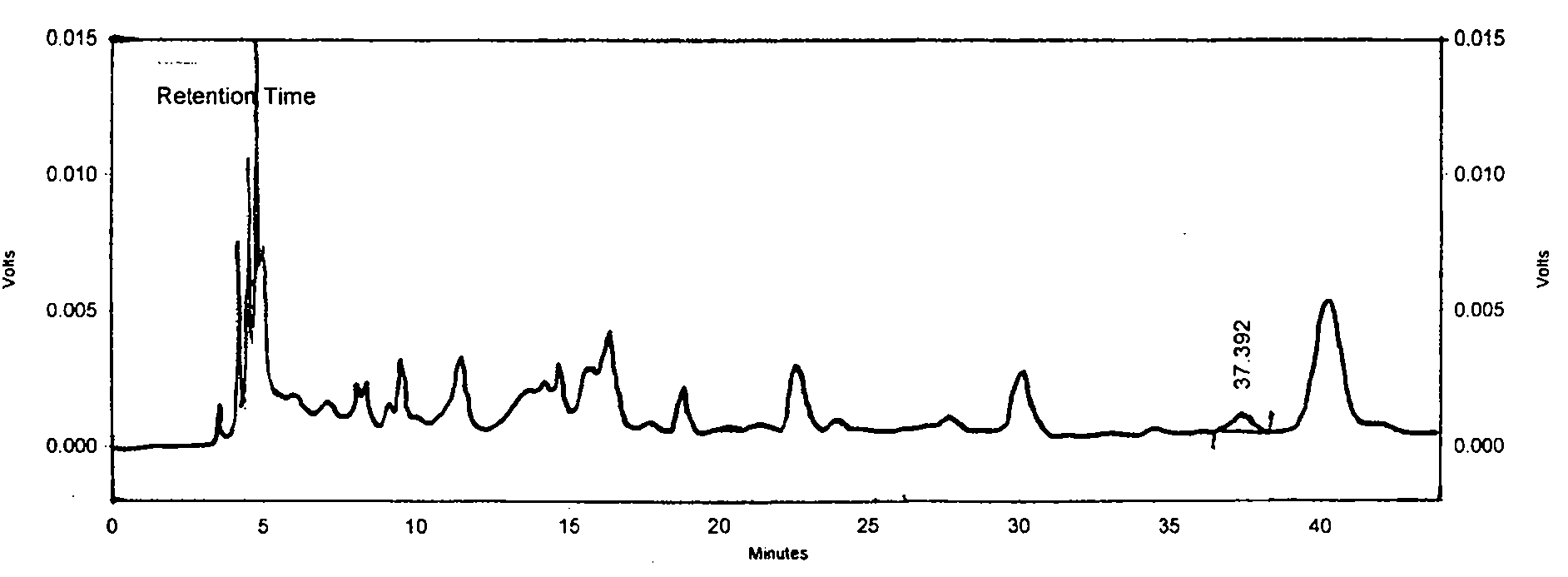
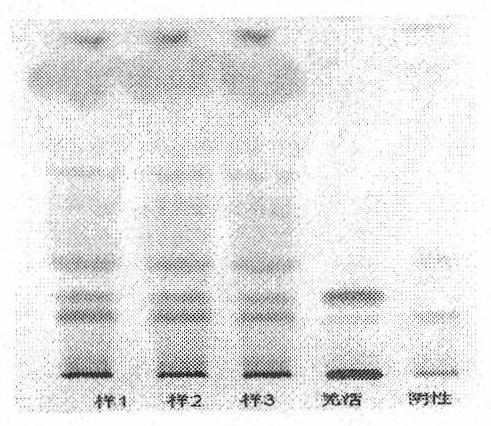
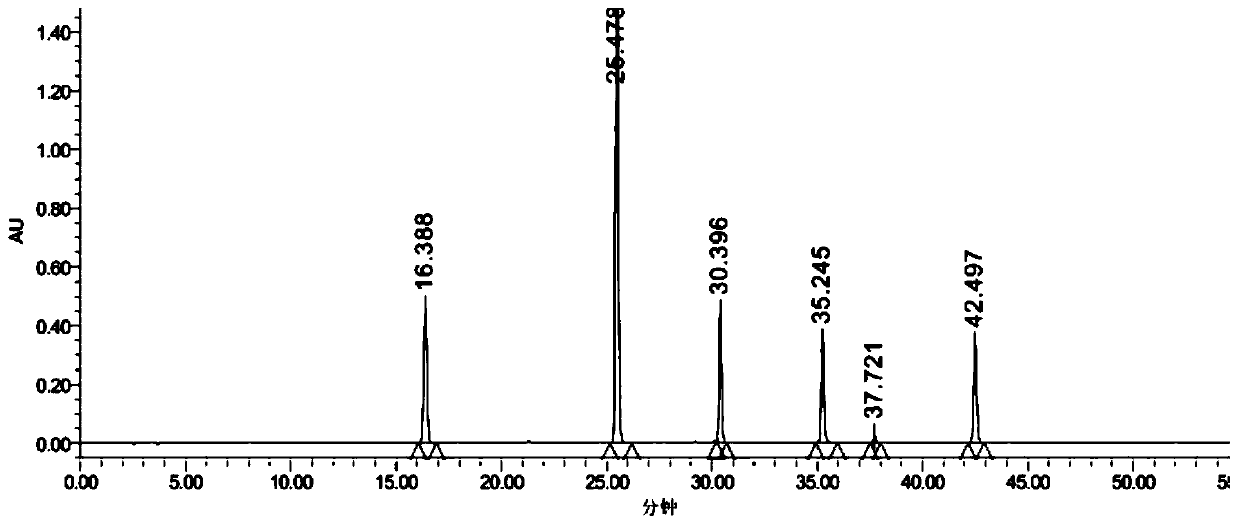
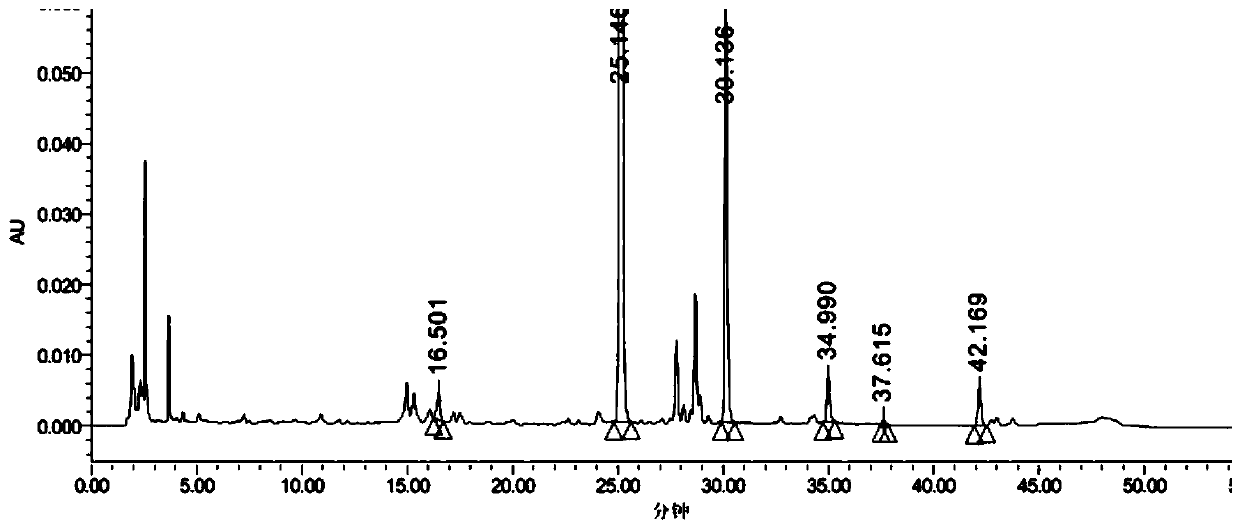
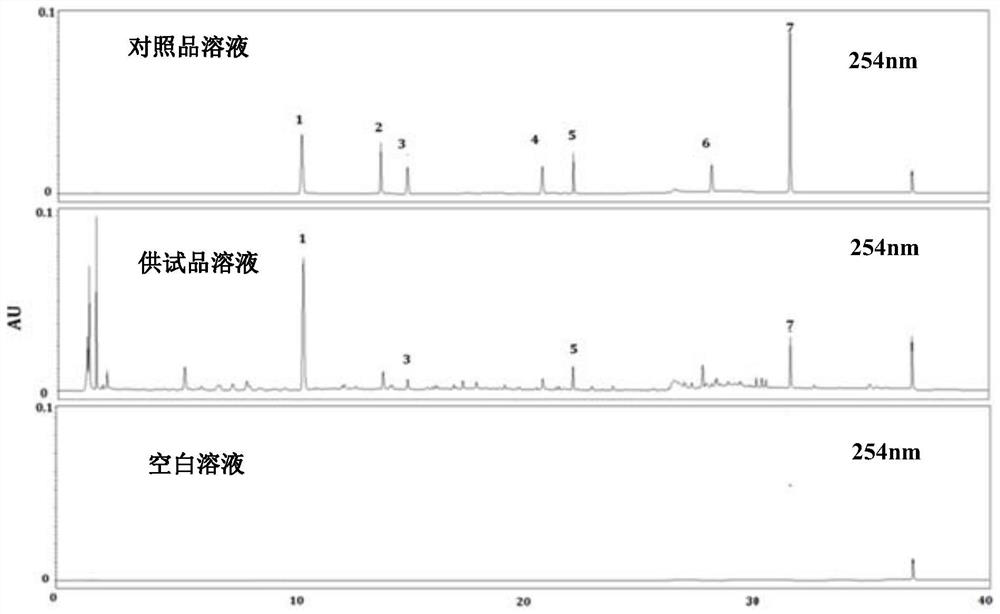
Patents
Literature
119 results about "Ammonium Glycyrrhizinate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
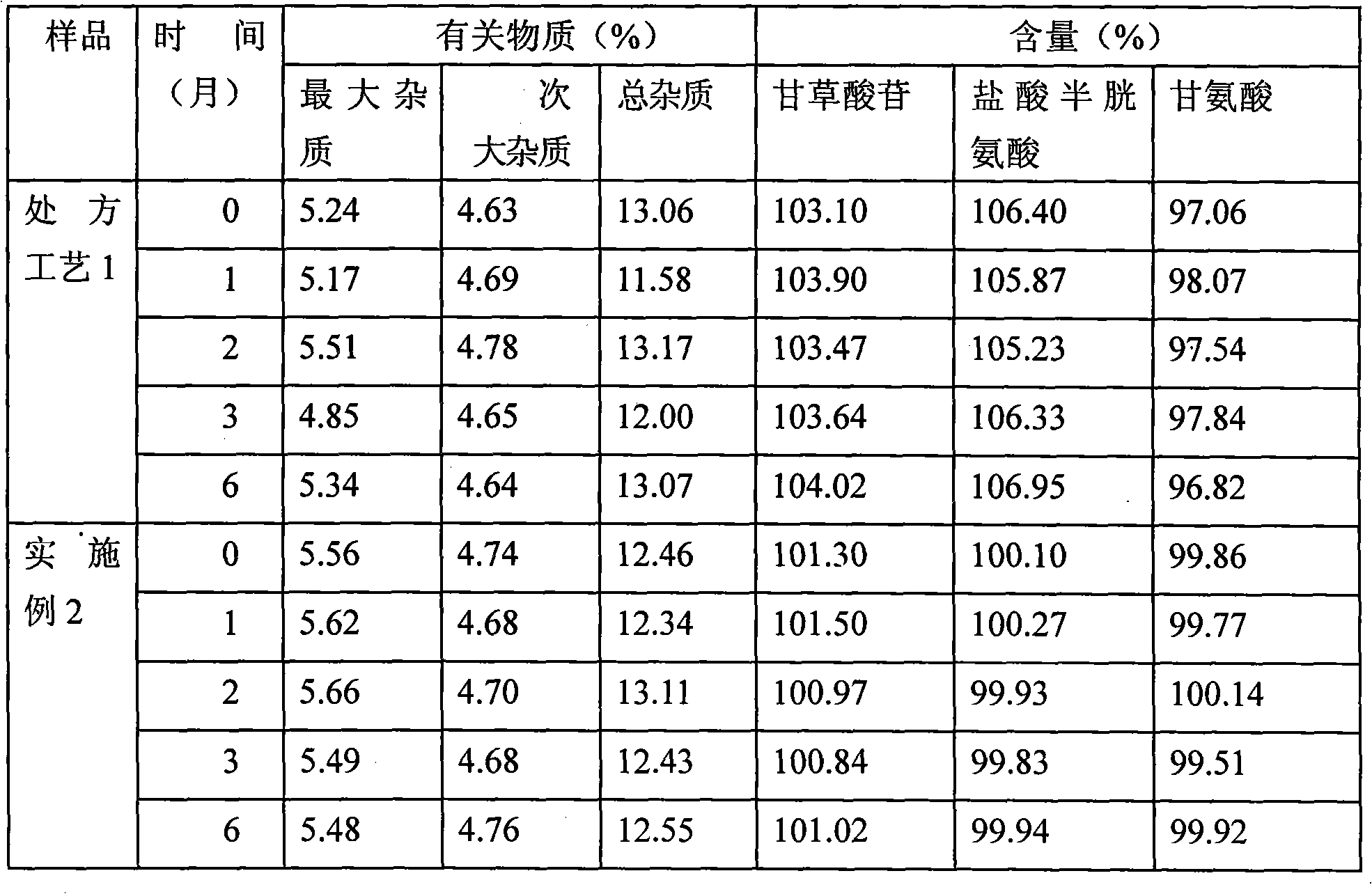
Mono ammonium Glycyrrhizinate. Glycyrrhizin (or glycyrrhizic acid or glycyrrhizinic acid) is the chief sweet-tasting constituent of Glycyrrhiza glabra (liquorice) root. Uses. Glycyrrhizin inhibits liver cell injury and is given intravenously for the treatment of chronic viral hepatitis and cirrhosis in Japan.
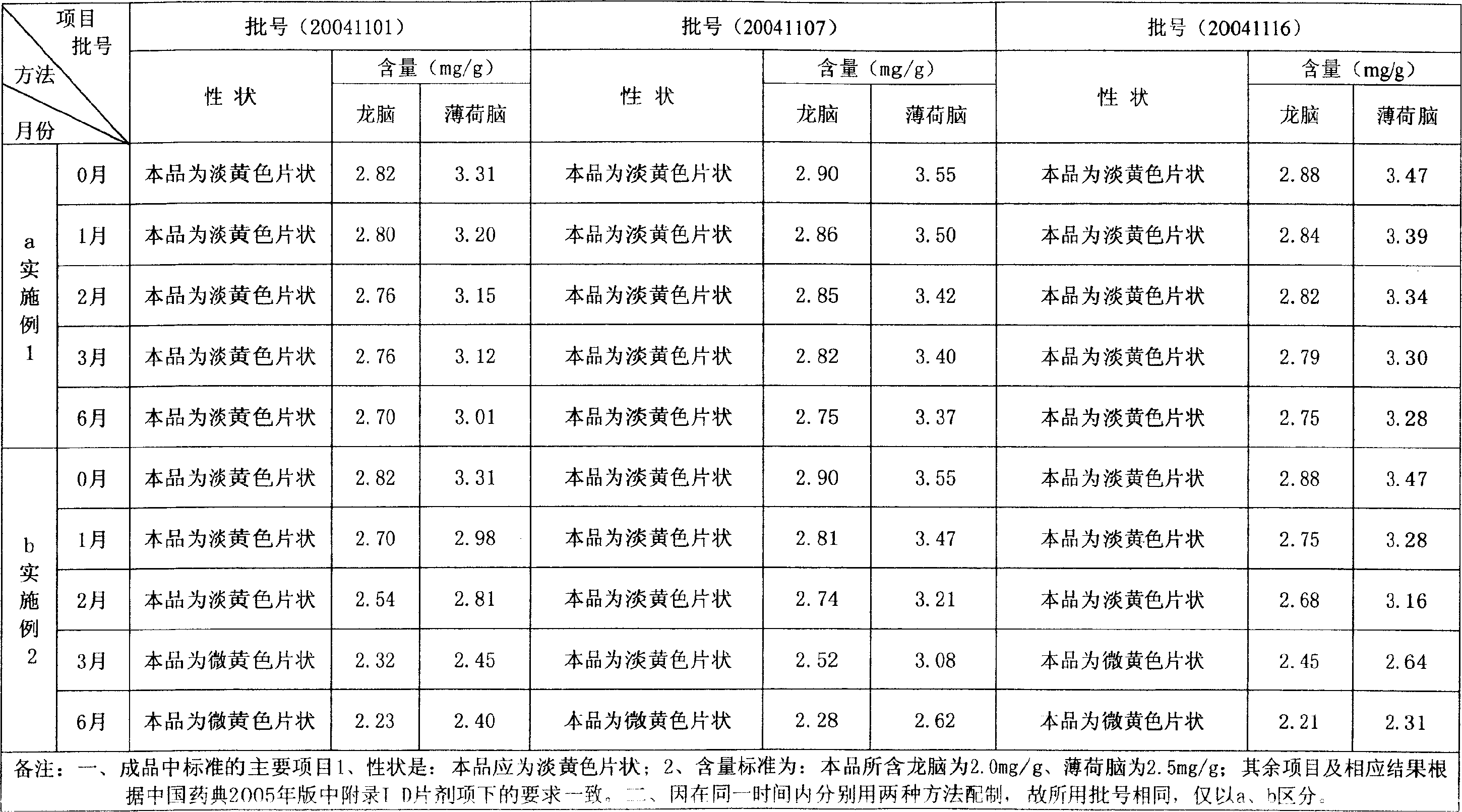
Quality detection method of traditional Chinese medicine preparation
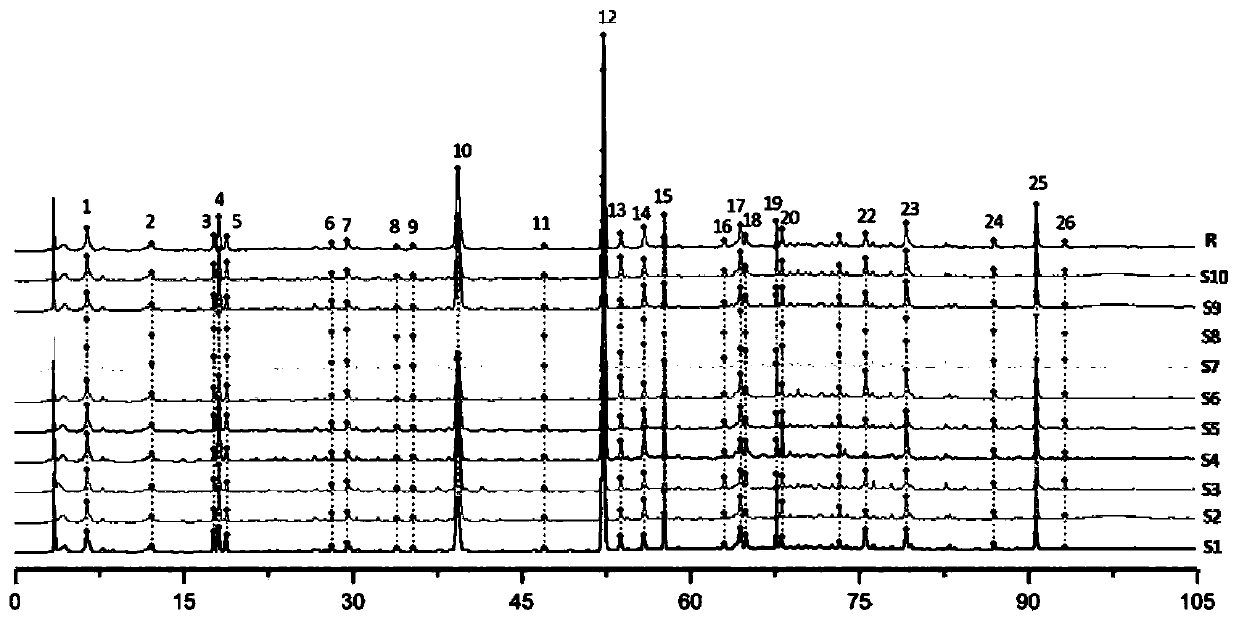
ActiveCN111044624AReasonable quality inspectionEasy to separateComponent separationBiotechnologyGallic acid ester
The invention relates to a quality detection method of a traditional Chinese medicine preparation. The method comprises the following steps: respectively taking gallic acid, albiflorin, paeoniflorin,ferulic acid, liquiritin, beta-ecdysterone, senkyunolide I, glycyrrhizin, cinnamic acid, cinnamyl aldehyde, paeonol, ammonium glycyrrhizinate and ligustilide as reference substances, and establishinga fingerprint spectrum of the traditional Chinese medicine preparation by adopting high performance liquid chromatography; identifying Chinese angelica, the rhizome of chuanxiong, radix paeoniae alba,moutan bark, ginseng, cassia bark, licorice root and the root of bidentate achyranthes of the traditional Chinese medicine preparation by adopting thin-layer chromatography. The traditional Chinese medicine preparation is prepared by using the following raw materials: Chinese angelica, the rhizome of chuanxiong, radix paeoniae alba, cassia bark, moutan bark, zedoray rhizome, ginseng, licorice root and the root of bidentate achyranthes. With the two detection ways, comprehensive quality evaluation can be conducted on the meridian-warming decoction traditional Chinese medicine preparation. Themethod is simple, convenient, high in accuracy and high in reproducibility; a scientific basis can be provided for quality detection and evaluation of the meridian-warming decoction traditional Chinese medicine preparation, and the product quality is effectively controlled.
Owner:GUANGZHOU BAIYUNSHAN ZHONGYI PHARMA COMPANY
Process for extracting purified glycyrrhizic acid from licorice residue
Owner:NORTHEAST FORESTRY UNIVERSITY +1
Method for preparing mono-ammonium glycyrrhizinate
The invention discloses a method for preparing mono-ammonium glycyrrhizinate, consisting essentially of the following processing steps of: dissolving glycyrrhizic acid powder into organic solvent solution for refluxing extraction; adding ammonia or ammonia water; and obtaining the mono-ammonium glycyrrhizinate after filtering and drying. The preparation method takes the glycyrrhizic acid powder as raw material to directly prepare mono-ammonium glycyrrhizinate, the operation is simple and convenient, the time is saved, the cost is reduced, and the product content is high, thus avoiding the drawbacks in the existing preparation process of the mono-ammonium glycyrrhizinate such as long steps, high cost, use of a large amount of organic solvent, not being beneficial to environmental protection, low product content, and the like.
Owner:XINJIANG FUWO PHARMA
Preparation method of mono-ammonium glycyrrhizinate
Owner:JIANGSU TIANSHENG PHARMA
Method for controlling quality of Longdanxiegan Capsule
ActiveCN101537088AScientific and perfect quality controlQuality improvementComponent separationDigestive systemAdjuvantGentiakochianin
The invention discloses a method for controlling the quality of the Longdanxiegan Capsule. The invention adopts the combined methods of character and thin layer chromatography discrimination and high efficiency liquid chromatography content measurement so as to control the quality of the Longdanxiegan Capsule, wherein during the operation of thin layer chromatography discrimination, characteristic spots of bupleurum, radix scutellariae, scutelloside, ferulic acid, glycyrrhiza and ammonium glycyrrhetate are respectively detected, the content of gentiamarin and geniposide is detected simultaneously by the high efficiency liquid chromatography under the condition of identical chromatogram through the mode of gradient elution, the content of the gentianella of each capsule is more than 0.07 mg according to gentiamarin, and the content of the gardenia of each capsule is more than 1.0 mg according to geniposide. The method respectively carries out the operation of thin layer chromatography discrimination on the characteristic components of the Longdanxiegan Capsule, can scientifically and comprehensively reflect the existence of monarch drugs, ministerial drugs, adjuvant and conductant drugs in the Longdanxiegan Capsule, simultaneously detects the content of the characteristic components of the gentianella and the gardenia; moreover, the method is simple, scientific and easy to operate, and is conducive to the comprehensive quality control of the Longdanxiegan Capsule.
Owner:成都尚科药业有限公司
Powder injection of compound glycyrrhizic acid glycosides and preparation method thereof
The present invention relates to compound glycyrrhizin type powder injection and the preparation method thereof, in particular to compound glycyrrhizin for injection, compound glycyrrhizic acid mono-ammonium S powder injection and the preparation method thereof, which are characterized in that the powder injection consisting of glycyrrhizin (or mono-ammonium glycyrrhizinate), glycine and cysteine hydrochloride that are taken as the active ingredient and the bearer acceptable in medicine and the preparation method are included in the prescription; wherein, the bearer acceptable in medicine contains dextran. The compound glycyrrhizin type powder injection of the present invention can be preserved at room temperature, thus remarkably improving the stability of the medicine, better guaranteeing safety and significance of the medicine and effectively decreasing the storage and transportation cost in production and transportation processes of the medicine.
Owner:TIBET WEIXINKANG MEDICINE CO LTD
Pleasant-tasting aqueous liquid composition of prednisolone sodium phosphate
A liquid pharmaceutical composition is contemplated that comprises a pharmaceutically effective amount of prednisolone sodium phosphate (PSP) dissolved or dispersed in an aqueous medium that is free of ethanol. The aqueous medium consists essentially of water, about 3 to about 10 weight percent polyvinylpyrrolidone, about 60 to about 75 weight percent of a C3-C6 polyol that includes more than 55 weight percent non-reducing disaccharide or trisaccharide such as sucrose, about 0.01 to about 0.5 weight percent ammonium glycyrrhizinate and one or more flavorants, and preferably includes one or more preservatives. The liquid composition is transparent and has a pleasant taste.
Owner:ASCENT PEDIATRICS
Mono-ammonium glycyrrhizinate and preparing method thereof
ActiveCN105237609AReduce contentEfficient use ofSugar derivativesSteroidsActivated carbonAqueous solution
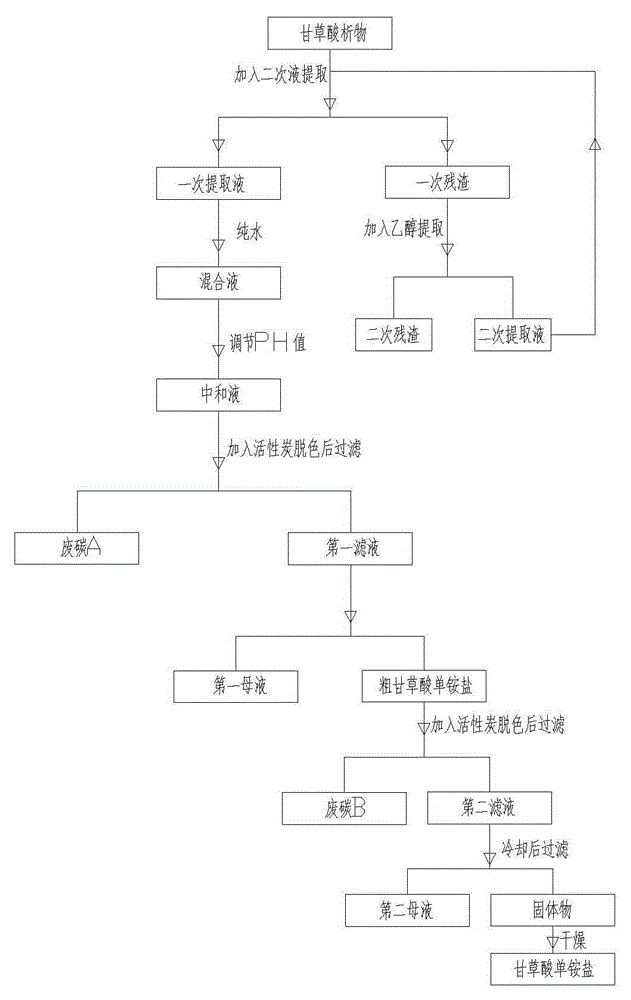
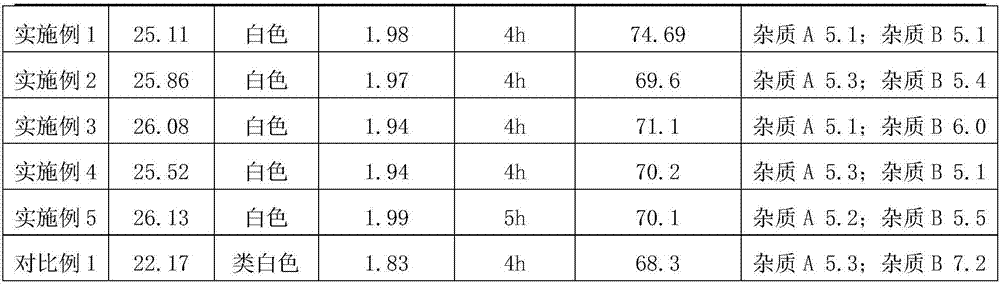
The invention relates to the technical field of mono-ammonium glycyrrhizinate, in particular to mono-ammonium glycyrrhizinate and a preparing method thereof. The preparing method comprises the following steps that firstly, a secondary extracting solution is added into glycyrrhiza acidification precipitate for extraction, and a primary extracting solution and primary residues are obtained through filtering after extraction; secondly, pure water is added in the primary extracting solution to adjust the water content of a mixed solution, and then the PH value of the mixed solution is adjusted; thirdly, active carbon is added into a neutralizing solution for decoloration, and a first mother solution and coarse mono-ammonium glycyrrhizinate are obtained through crystallization after decoloration; fourthly, ethanol water and active carbon are added into the coarse mono-ammonium glycyrrhizinate for decoloration, and a second mother solution and the mono-ammonium glycyrrhizinate are obtained through crystallization after decoloration. Compared with an existing technology, by means of the preparing method, the production cost is greatly reduced, and resources are effectively utilized; meanwhile, compared with mono-ammonium glycyrrhizinate obtained through the existing technology, the prepared mono-ammonium glycyrrhizinate is low in content of impurities A, lighter in color and better in quality.
Owner:新疆天山制药工业有限公司
Pore refining essence and production method thereof
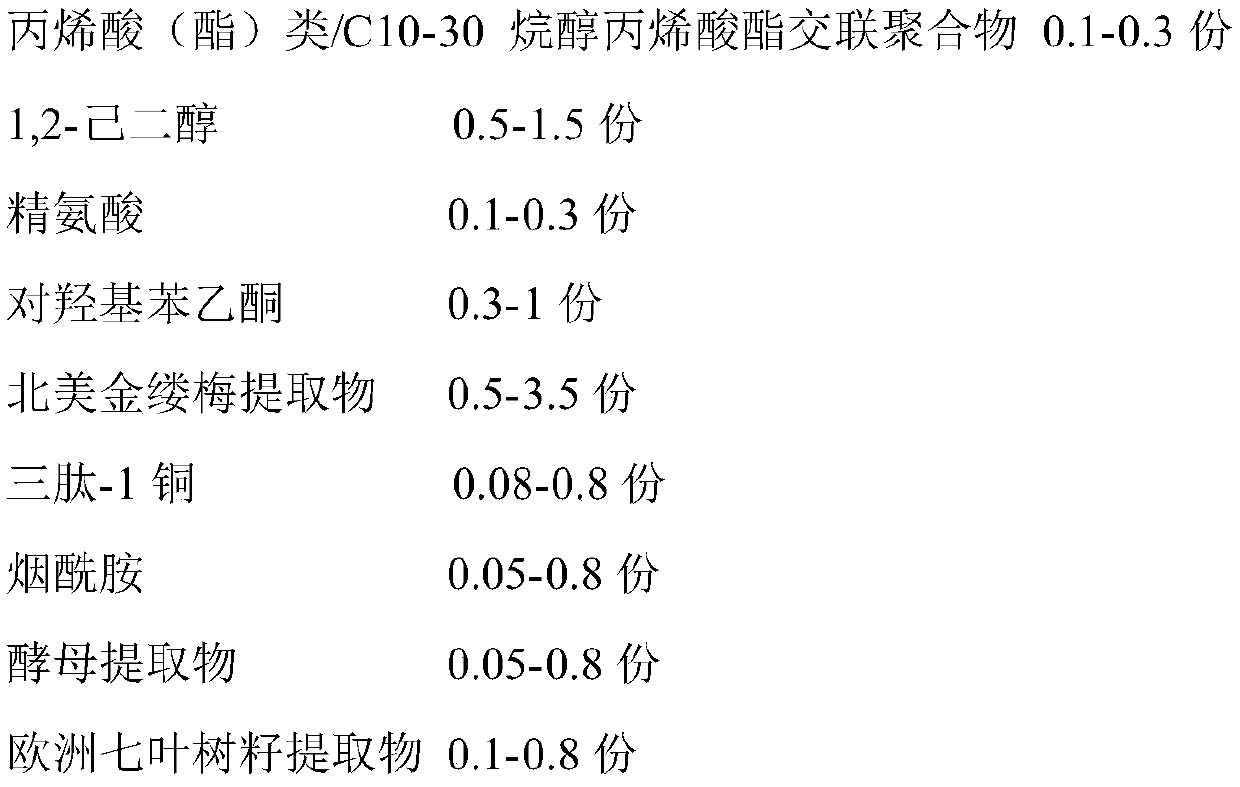
InactiveCN111084743AImprove immediate efficacyReduce bulkCosmetic preparationsToilet preparationsBiotechnologyArginine
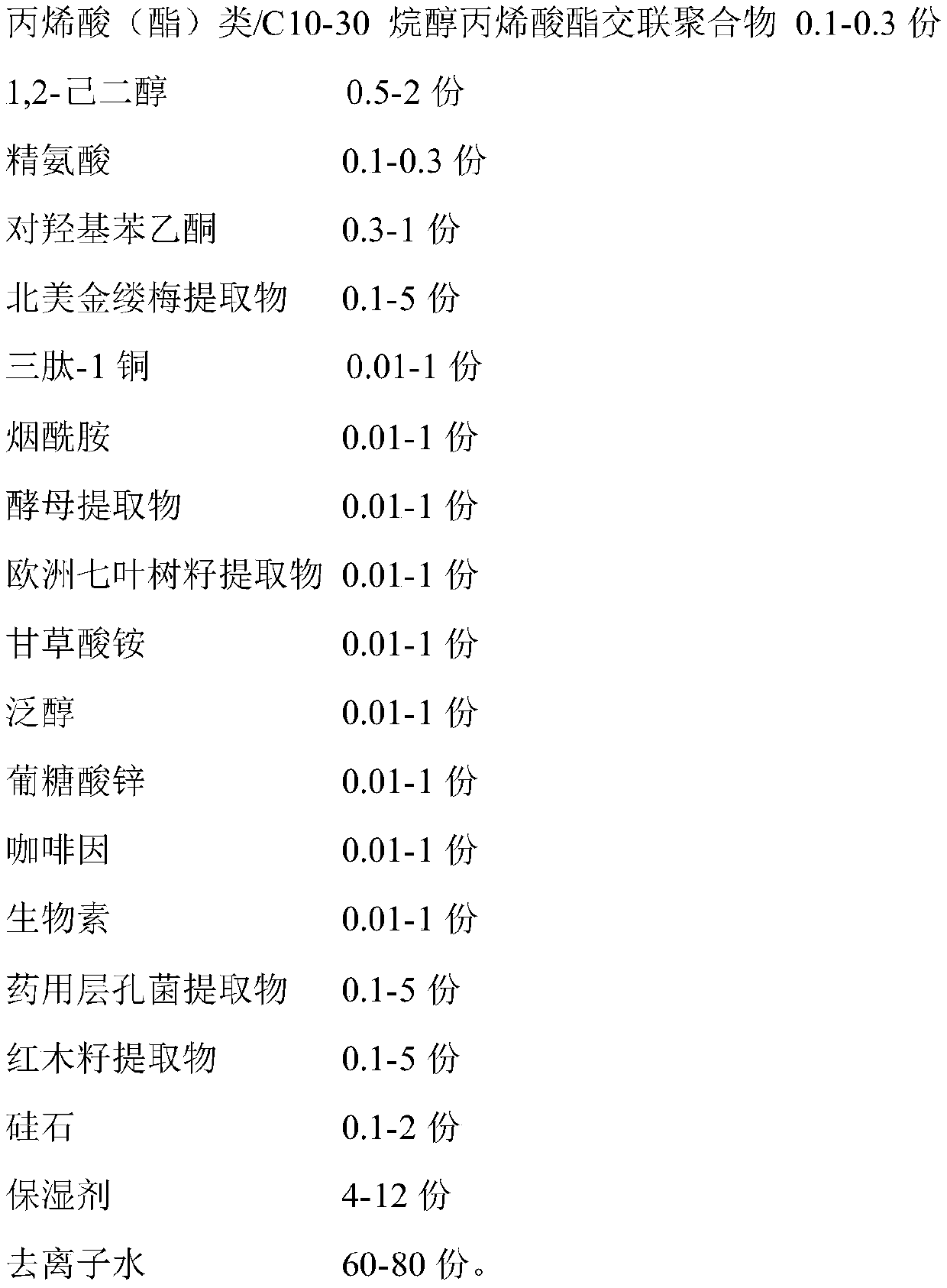
The invention relates to the technical field of skin care products, in particular to pore refining essence and a production method thereof. A formula of the pore refining essence is mainly composed ofthe following components in parts by weight: 0.5-2 parts of dl-1,2-hexanediol, 0.1-0.3 part of acyclic acid (acrylate) / C10-30 alkyl acrylate cross-linked polymer, 0.1-0.3 part of arginine, 0.3-1 partof 4'-hydroxyacetophenone, 0.1-5 parts of a hamamelis virginiana extract, 0.01-1 part of glycyl-l-histidyl-l-lysine, 0.01-1 part of nicotinamide, 0.01-1 part of an aesculus hippocastanum seed extract, 0.01-1 part of a glycyrrhizic acid ammonium salt, 0.1-5 parts of a medicinal fomes officinalis extract, 0.1-5 parts of a bixa orellana seed extract, 0.1-2 parts of silica, 2-13 parts of a moisturizer and the like. According to the pore refining essence, not only is the instant efficacy of pore refining and sebum clearing improved to alleviate the problem of coarse pores from the origin, but alsomultiple aspects of pore cleaning, oil-water balance adjustment, repairing, moisturizing and the like are taken into account to repair skin and improve skin conditions fundamentally, and the pore refining essence is safe and non-irritant.
Owner:广州果壳生物科技有限公司
Medicine preparation for treating oral cavity and larynx disease
InactiveCN1927268AEasy to useSatisfying demand willing to purchase small dose packagingHydroxy compound active ingredientsAerosol deliveryDiseaseThroat
This invention relates to a new praeparatum of drug King-hour jian for the treatment of oral and throat diseases. It takes balsamiferous biumea leaf and branchlet oil, pungent litse fruit oils, menthol and ammonium glycyrrhizinate salt for raw materials, manufactured into three agents: dripping pills, buccal tablets, and aerosol. The invention expands the serviceable range of King-hou jian drug, can meet the needs of different groups of people, satisfy different favor of different people in pharmaceutical dosage form, and facilitate patients to choose the pharmaceutical dosage form, with more convenient for the use of patients, more direction and better herapeutic effect.
Owner:贵州宏宇药业有限公司
Preparation method of diammonium glycyrrhizinate salt capable of accurately controlling ammonium radical content
ActiveCN101914126AHigh yieldSolve the problem of wrong root contentSugar derivativesSteroidsDiammonium GlycyrrhizinateProtic solvent
The invention relates to a preparation method of a diammonium glycyrrhizinate salt capable of accurately controlling ammonium radical content, which is characterized by comprising the following steps of: using a mono-ammonium glycyrrhizinate salt as an initiative raw material; dissolving by adding a protic solvent; regulating a pH value as 4.5-9.0 by adding ammonia; drying by using a suitable method; and monitoring the ammonium radical content by adopting indophenol blue colorimetry to consequently prepare the diammonium glycyrrhizinate salt with correct ammonium radical content. A preparation technology and a monitoring method of the invention solve the problem of wrong ammonium radical content of the diammonium glycyrrhizinate salt prepared by the prior art in a breakthrough mode, reduce crystallizing steps at the same time, greatly increase the diammonium salt yield and are suitable for industrialized production. The prepared diammonium glycyrrhizinate salt also has definite18alpha and 18beta configurational compositions.
Owner:BEIJING UNION PHARMA FACTORY
Spray for preventing and treating skin damnification and resisting infection
InactiveCN101584715AGood curative effectSmall side effectsOrganic active ingredientsAnthropod material medical ingredientsPropolisCurative effect
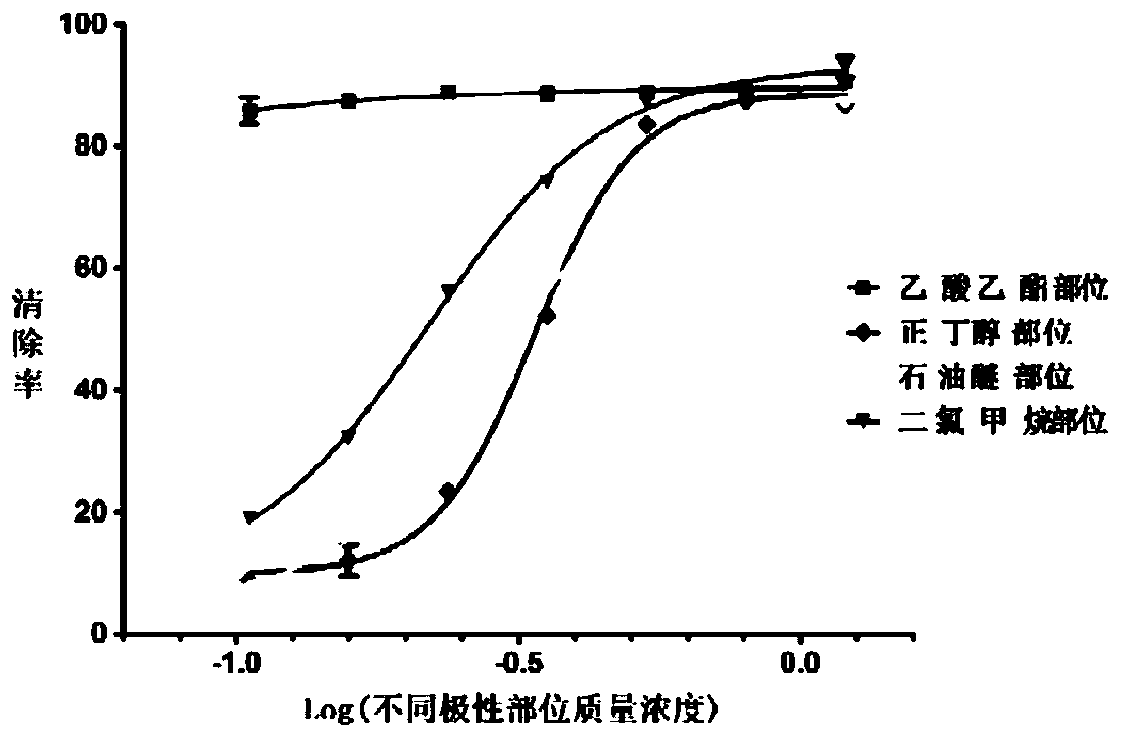
The invention discloses a spray for preventing and treating the skin damnification and resisting the infection, belonging to the Chinese medicine preparation field. The spray is prepared from the following components based on the weight percent: 0.2 to 5% of bee glue, 1 to 20 % of 95%ethanol, 3 to 20% of tween 80, 0.04 to 0.3 % of borneol, 0.5 to 8% of mono-ammonium glycyrrhizinate and the balance of distilled water. the medicament of the invention is a Chinese medicine spray for preventing and treating the skin damnification and resisting the infection. The spray has stabilizing curative effect, little toxic side effect, safe use and lower treating cost.
Owner:THE FIFTH MEDICAL CENT OF CHINESE PLA GENERAL HOSPITAL
Quality control method for infantile cough syrup as Chinese medicine preparation
ActiveCN102068626AImprove quality controlRaise quality standardsComponent separationRespiratory disorderLicorice acidMedicine
The invention belongs to the technical field of Chinese patent drug, relating to a quality control method for infantile cough syrup as Chinese medicine preparation, which is prepared from Chinese herbal medicines as raw materials, comprising the following steps: with the reference substances of figwort, lilyturf root and Sarsasapogenin, distinguishing whether the prescription of the infantile cough syrup contains the compounds of figwort, lilyturf root and Sarsasapogenin with thin layer chromatography; and with the reference substance of mono-ammonium glycyrrhizinate, detecting the content of glycyrrhizic acid in the prescription of the infantile cough syrup with high performance liquid chromatography; therefore, the quality standard is relatively perfect, and the quality control of the medicine is improved due to the amended quality standard.
Owner:津药达仁堂集团股份有限公司乐仁堂制药厂
Preparation method of beta-mono-ammonium glycyrrhizinate
The invention provides a preparation method of beta-mono-ammonium glycyrrhizinate. According to the preparation method, the beta-mono-ammonium glycyrrhizinate is prepared by carrying out a series of steps such as crushing of beta-glycyrrhizic acid serving as a raw material, sieving, solid-state reaction, extracting, concentration and drying, and particularly utilizing a key technology that beta-glycyrrhizic acid powder can react with ammonia gas in a certain particle size range to generate the water-soluble beta-mono-ammonium glycyrrhizinate. Compared with a traditional preparation technology, the preparation method provided by the invention has very strong practicability and the advantages of energy conservation, environmental protection, low production cost and the like and is suitable for industrial production.
Owner:HUAIAN BROTHER BIOLOGICAL TECH
Production method of glycyrrhizin
The invention provides a production method of glycyrrhizin, which comprises the following steps: S1. pulverizing licorice, soaking in 30-45 DEG C water for 6-36 hours, taking the leach solution, concentrating to paste, drying, and pulverizing to obtain acid powder; S2. leaching the acid powder with ethanol, aminating the leach solution with ammonia water or ammonia gas to obtain a yellow precipitate-triammonium glycyrrhizinate, and separating out the triammonium glycyrrhizinate; and S3. acidifying the triammonium glycyrrhizinate with glacial acetic acid, heating under reflux for 0.5-2 hours, standing, and separating out the mono-ammonium glycyrrhizinate crude product. The method is performed by using heavy-metal-free raw materials and equipment, and optimizes the technological parameters.
Owner:毛林涛
Diammonium glycyrrhizinate salt preparation method
The invention discloses a diammonium glycyrrhizinate salt preparation method. According to the method, according to the special physical and chemical properties of glycyrrhizic acid, the diammonium glycyrrhizinate salt with characteristics of good color, good character, high content and meeting of the industry standard is produced by using an ammonium glycyrrhizinate salt as a raw material. Compared to the method in the prior art, the method of the present invention has significant advantages of novel process, simple operation, energy saving, environmental protection, strong practicality, mild reaction condition, high conversion rate, easy quality control, reduction of the use of other solvents, reduction of impurity introduction, good product color, simple operation process, low equipment and material cost, high treatment capacity, good product chrominance, good product yield, high content, excellent product color, uniform crystal and good quality, and is suitable for industrial production.
Owner:JIANGSU TIANSHENG PHARMA
Method of establishing fingerprint spectrum of infant diarrhea stopping drug preparation
ActiveCN106802327AImprove effectivenessImprove securityComponent separationDrugs preparationsPharmaceutical drug
The invention belongs to the field of drug analysis and particularly relates to a method of establishing a fingerprint spectrum of an infant diarrhea stopping drug preparation. According to the method, by taking the infant diarrhea stopping drug preparation as a detection object, the method of establishing the fingerprint spectrum of the infant diarrhea stopping drug preparation is established, common characteristic peaks including peak 1, peak 2 liquiritin, peak 3 ammonium glycyrrhizinate, peak 4, peak 5, peak 6, peak 7 and peak 8 are affirmed, the peak 2 liquiritin is selected as an internal reference peak in the fingerprint spectrum, and a relative retention time of each common characteristic peak is determined; and in addition, with combination of information of a plurality of chromatographic peaks in the fingerprint spectrum, the quality of the infant diarrhea stopping drug preparation can be comprehensively and rapidly detected, so that comprehensive quality detection and whole quality control of the infant diarrhea stopping drug preparation are facilitated, and improvement on the use safety and stability of the drug is facilitated. Simultaneously, the method of establishing the fingerprint spectrum of the infant diarrhea stopping drug preparation has the advantages of high precision, good repeatability, high stability and the like.
Owner:合肥华润神鹿药业有限公司
Compound ammonium glycyrrhizinato S dispersed tablet and its preparing process
InactiveCN1919185AImprove complianceSimple production processOrganic active ingredientsDigestive systemGlycineDL-methionine
The invention discloses a compound ammonium glycyrrhizinate S dispersible tablet, wherein the dispersible tablet is prepared from the following raw materials (by weight portion): monoammonium glycyrrhizinate S (calculated by glycyrrhizinic acid) 5-30 parts, glycine 5-30 parts, DL-methionine 5-30 parts, bulking agent 55-220 parts, crumbling agent 32-160 parts, lubricating agent 0.2-5 parts. The dispersible tablet has the advantages of shorter disintegration time, better dispersion state, faster medicament dissolving, quicker absorption, and higher biological availability, thus is especially suitable for the elder people and patients suffering from swallowing difficulty.
Owner:黄本东
Quality control method of traditional Chinese medical preparation
ActiveCN102038869AStrong process controllabilityAdd TLC identification test methodAnthropod material medical ingredientsAntipyreticDrug standardsThin-layer chromatography
The invention relates to a quality control method of a traditional Chinese medical preparation. The method comprises the following steps of: firstly, carrying out microscopic identification; secondly, identifying whether a juvenile wind-transmission pill prescription contains the contents of notopterygium root, radix sileris, rhizome of chuanxiong, ginseng and liquorice or not by adopting a film chromatography; and finally, measuring the liquorice content in the juvenile wind-transmission pill prescription by adopting a high-efficiency liquid-phase method and using ammonium glycyrrhetate as a reference substance. Based on an original standard of the traditional Chinese medical set prescription preparation volume III of the drug standard of the health department, the detection method provided by the invention adds the film identification detection methods of reference medical materials and reference substances and a method for measuring the content by using a high-efficiency liquid-phase chromatography. The revised quality standard control method improves the controllability of the juvenile wind-transmission pill quality standard, further ensures the internal quality and the curative effect of the product and is significant to promote the product marketing, enhance the market competitiveness of the product and ensure the drug safety for patients.
Owner:津药达仁堂集团股份有限公司达仁堂制药厂
Quality detection method for traditional Chinese medicine pediatric cold-relieving granules
ActiveCN110736799ASimple and fast operationAnalytical data is accurateComponent separationBaicaleinFluid phase
The invention relates to a quality detection method for traditional Chinese medicine pediatric cold-relieving granules. According to the invention, the contents of baicalin, wogonoside, baicalein, wogonin, liquiritin and ammonium glycyrrhizinate in the traditional Chinese medicine pediatric cold-relieving granules are determined by high performance liquid chromatography. According to the invention, the separation degree between each to-be-detected component in the chromatogram of a test sample and the adjacent peak is greater than 1.5, and negative control has no interference, so that the quality safety of the granules is further ensured, evaluation is comprehensive, and the detection method has the advantages of high practicability, high operability, cost saving and the like in operation.
Owner:SHANDONG MINGREN FURUIDA PHARMA
Determination method for active ingredients of Juanbi decoction preparation
ActiveCN114200045AImprove stabilityGood repeatabilityComponent separationAgainst vector-borne diseasesPhosphoric acidGradient elution
The invention belongs to the technical field of medicinal preparation and traditional Chinese medicine ingredient detection, and particularly relates to a method for determining active ingredients of a Juanbi decoction preparation. The method comprises the following steps: firstly, preparing Juanbi decoction into granules, adopting an UPLC (Ultra Performance Liquid Chromatography) method, taking acetonitrile-0. 1% phosphoric acid water as a mobile phase, and carrying out gradient elution to simultaneously detect gentiopicroside, ferulic acid, decurtin, cinnamaldehyde, ammonium glycyrrhizinate, cnidium lactone and 11-carbonyl-beta-acetyl masticinic acid in the active ingredients of the Juanbi decoction as a classic famous formula compound preparation; the determination method provided by the invention has the advantages of good stability, good repeatability and good recovery rate, and can be used as a quality control means of the Juanbi decoction preparation.
Owner:SINOPHARM GUANGDONG GLOBAL PHARMA CO LTD
Anti-bacterial skin-care foot washing plant liquid and preparation method thereof
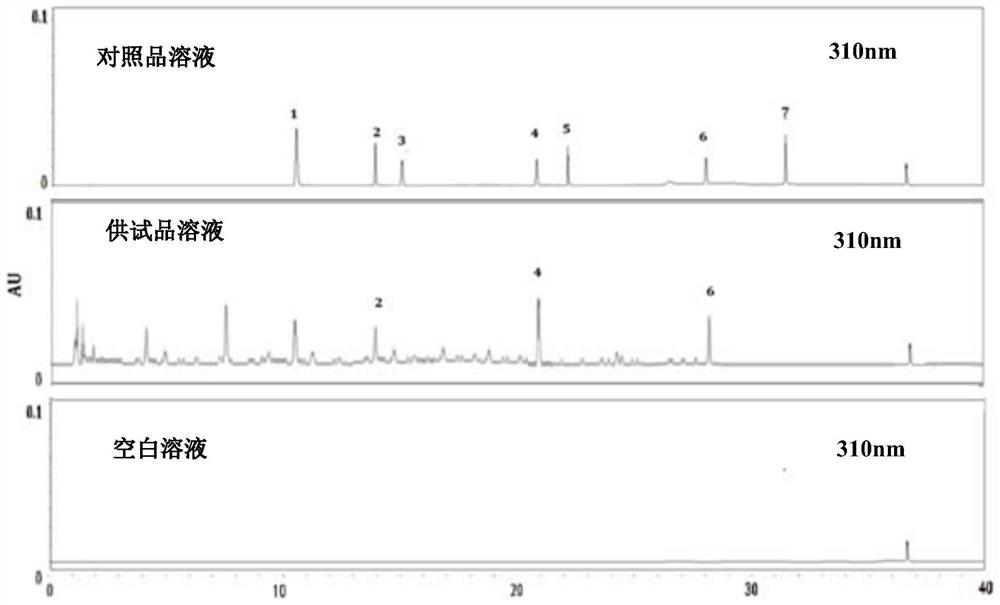
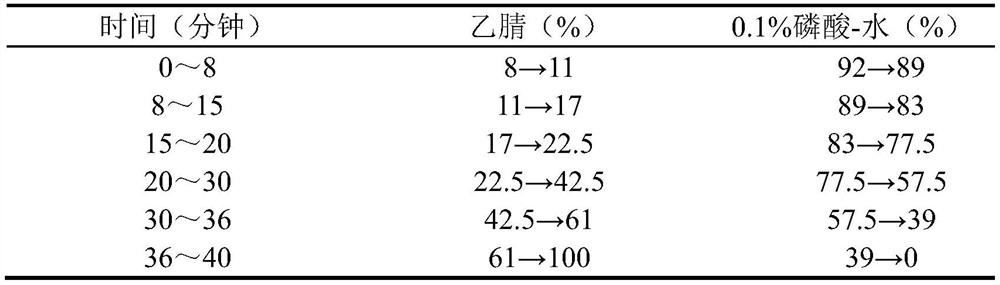
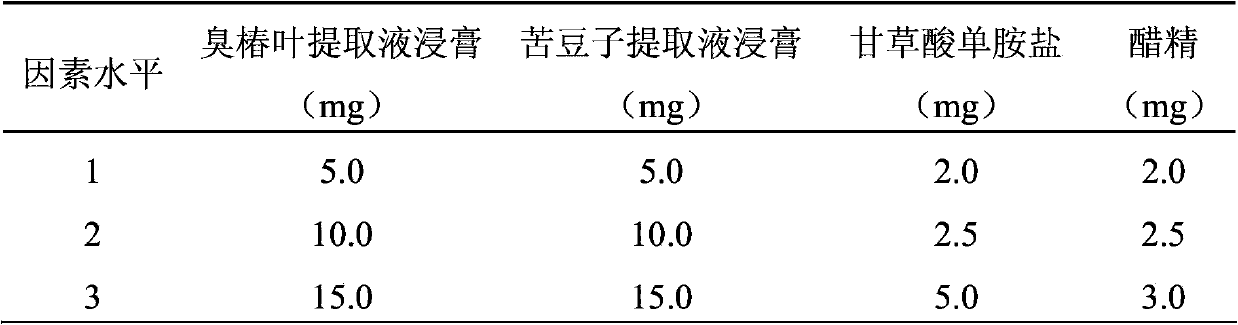
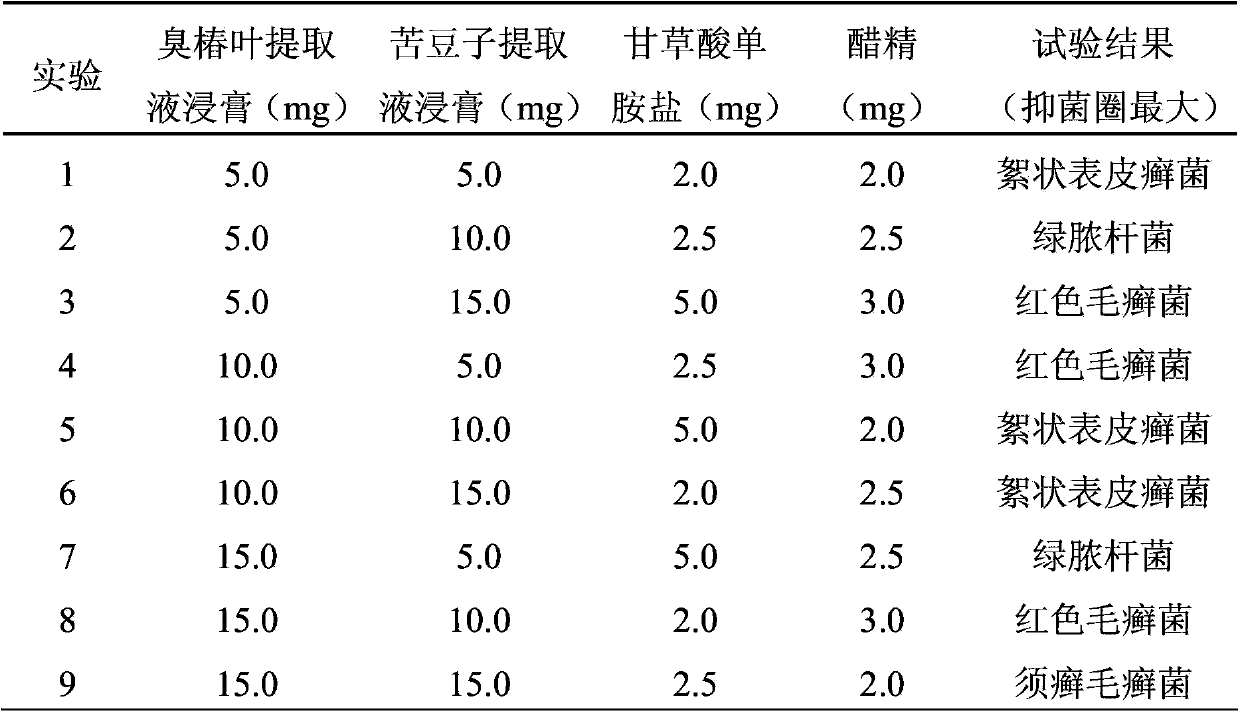
ActiveCN103417615AQuality improvementGood antibacterial effectAntibacterial agentsAntimycoticsAlcoholAilanthus
Anti-bacterial skin-care foot washing plant liquid comprises the following components: 5-15% of ailanthus leaf extract, 5-15% of sophora alopecuroide extract, 2-5% of ammonium glycyrrhizinate, 2-3% of acetin, 0.01-0.25% of preservative, 2-5% of antifreezing agent and the balance of deionized water. The preparation method of the anti-bacterial skin-care foot washing plant liquid comprises the following steps: (1) preparing the ailanthus leaf extract by adopting a water extraction and alcohol precipitation method; (2) putting the sophora alopecuroide in an extraction pot, adding 50% ethanol to the extraction pot, heating for refluxing for 2 h, and concentrating the mixture into the sophora alopecuroide extract, wherein the weight of the 50% ethanol is four times of that of the sophora alopecuroide; (3) dissolving the sophora alopecuroide extract, the sophora alopecuroide extract and the ammonium glycyrrhizinate into half amount of the deionized water, adding acetin to the mixture, so as to obtain medical liquid; (4) adding the preservative and refrigerant to the other half of the deionized water, and stirring the mixture until the mixture is uniform; (5) pouring the medical liquid to the deionized water containing the preservative and the refrigerant, stirring the mixture, placing the mixture still, sealing the mixture, and storing the mixture in shade. The anti-bacterial skin-care foot washing plant liquid has the advantages of stable quality, good bacteriostasis effect, low toxicity, high efficiency, simple preparation process and low cost.
Owner:广州玉婷阁医药科技有限公司
Ammonium glycyrrhizinate compound and pharmaceutical composition containing ammonium glycyrrhizinate
InactiveCN103613633AImprove solubilityFast dissolution rateOrganic active ingredientsAntipyreticSolubilityAmmonium compounds
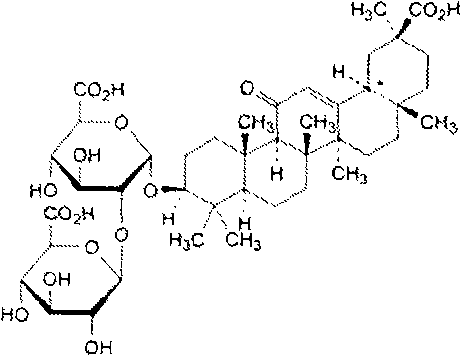
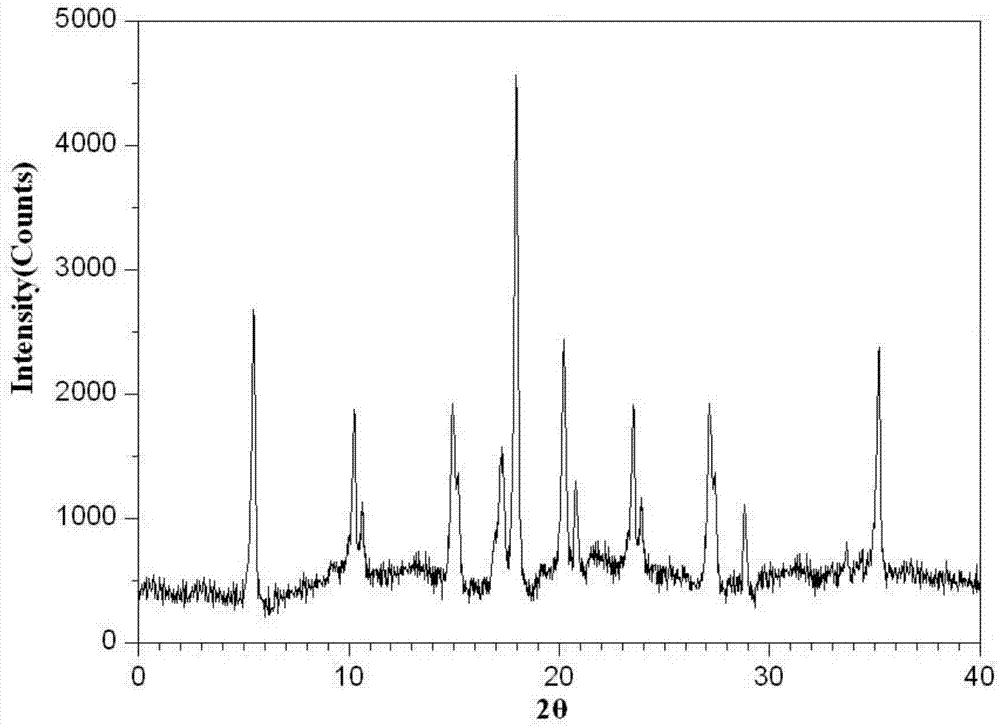
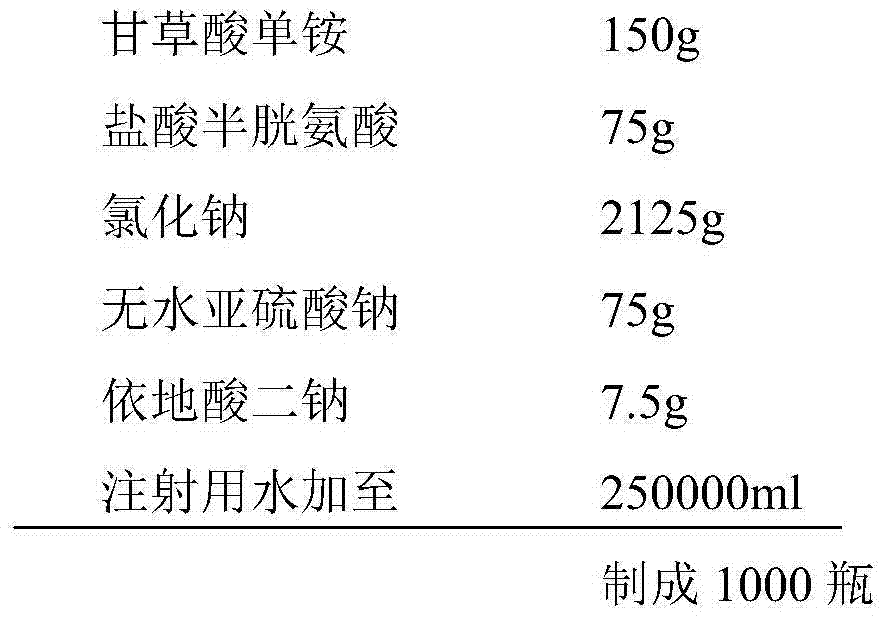
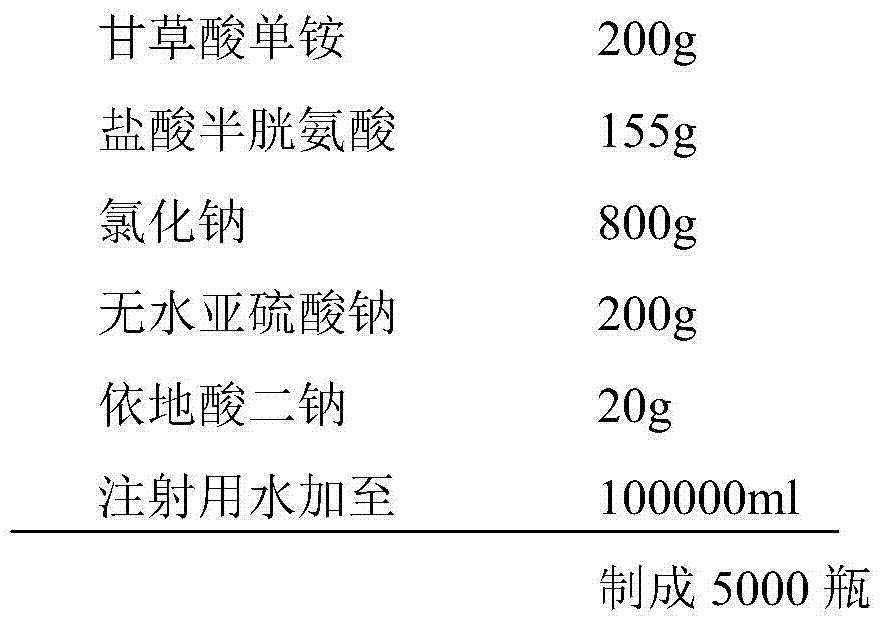
The invention belongs to the technical field of a medicine, and particularly relates to an ammonium glycyrrhizinate compound and a pharmaceutical composition containing the ammonium glycyrrhizinate compound. The ammonium glycyrrhizinate compound disclosed by the invention is crystal, and is determined by a powder X-ray diffraction measurement method, the molecular formula is C42H65NO16.2H2O; an X-ray powder diffraction pattern represented by a diffraction angle of 2theta+ / -0.2 degrees is shown in the figure 1. The pharmaceutical composition provided by the invention contains the ammonium glycyrrhizinate compound disclosed by the invention, cysteine hydrochloride and pharmaceutical acceptable auxiliary materials, and preferably comprises the following components in parts by weight: 1-200 parts of ammonium glycyrrhizinate, 1-200 parts of cysteine hydrochloride, 1-1,000 parts of sodium chloride, 1-200 parts of anhydrous sodium sulfite and 1-100 parts of edetate disodium. The ammonium glycyrrhizinate compound disclosed by the invention has good solubility in water, and is fast in dissolution rate, good in redissolution ability, and convenient to use, and the medication safety of a sufferer is greatly improved.
Owner:弘和制药有限公司
Levetiracetam oral solution and preparation method thereof
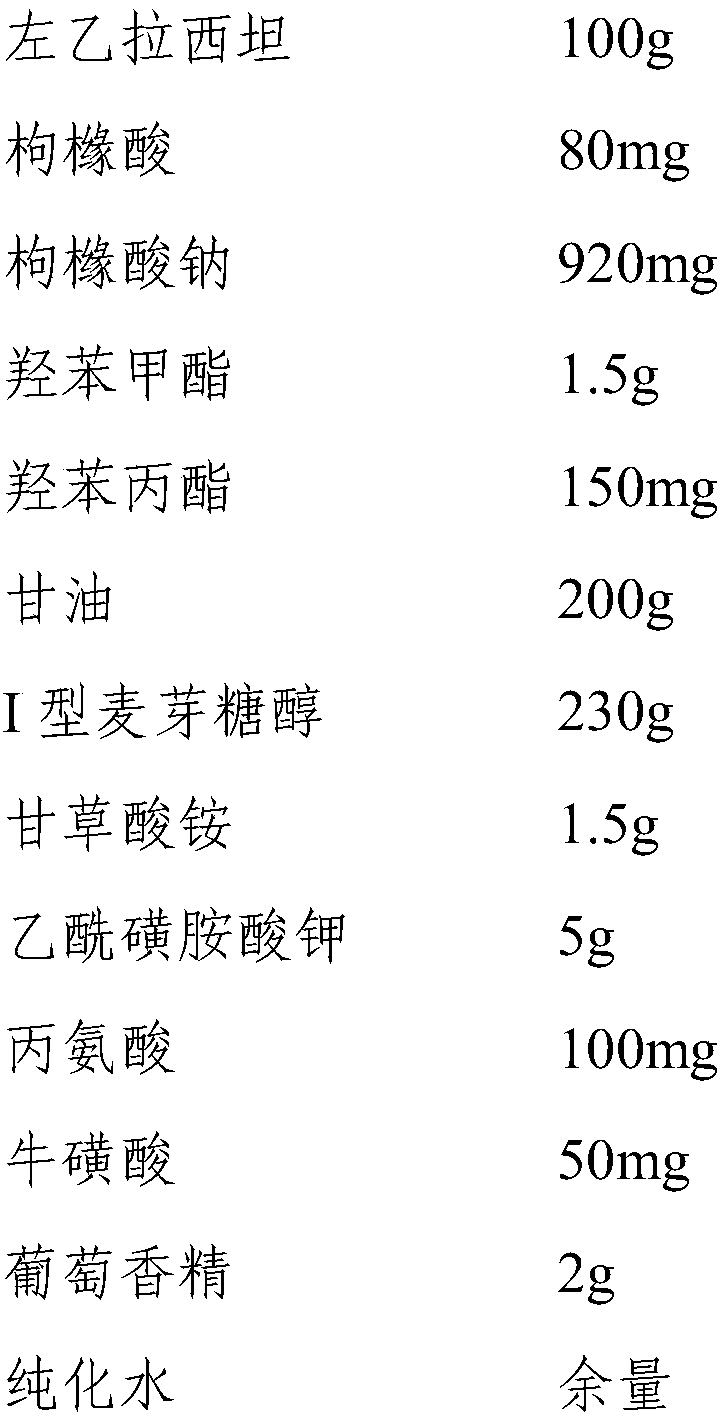
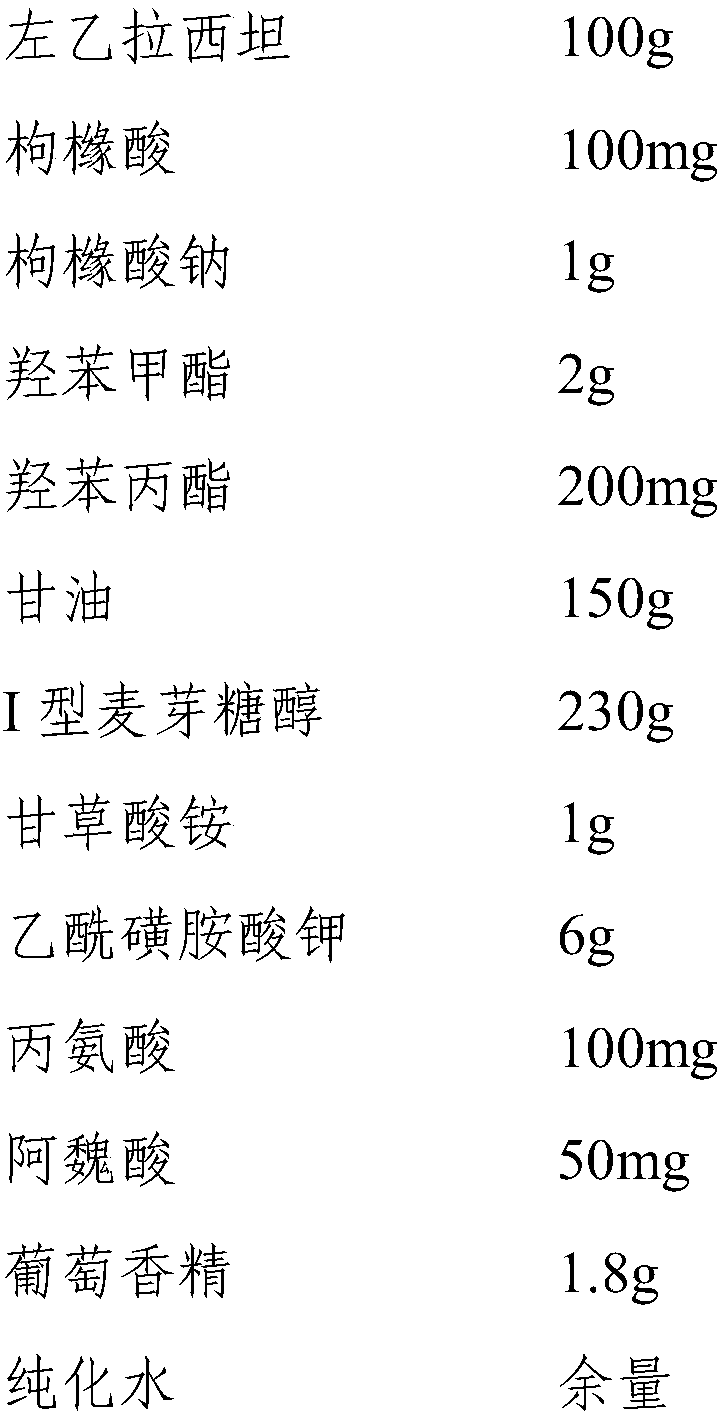
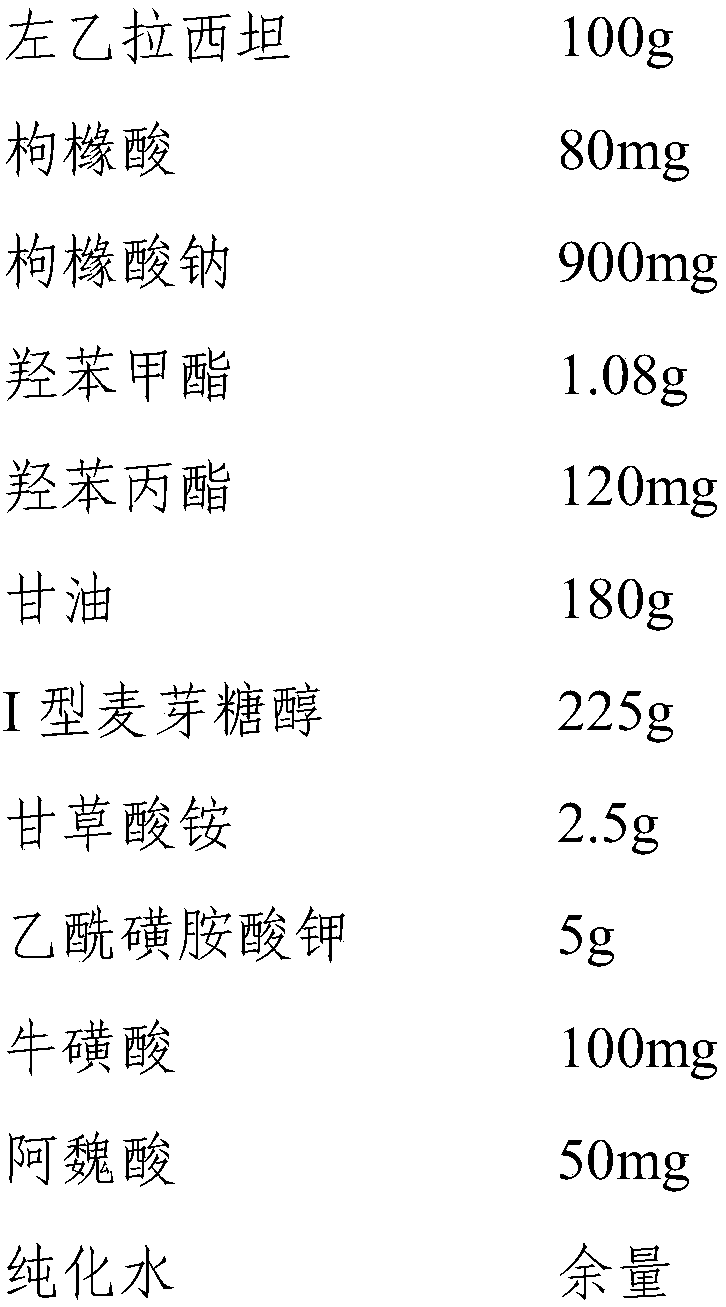
InactiveCN110193008ALow in preservativesMeet antibacterial effectOrganic active ingredientsNervous disorderGlycerolMaltitol
The invention discloses a levetiracetam oral solution and a preparation method thereof, wherein the levetiracetam oral solution comprises levetiracetam, a preservative, a flavoring agent, a buffer agent and a solvent. The content of levetiracetam is 10 g / 100 ml; the content of the preservative is 120-220 mg / 100 ml, and the preservative is a mixture of methylparaben and propylparaben; the content of the flavoring agent is 22.5-25 g / 100 ml, and the flavoring agent is a mixture of maltitol type I, ammonium glycyrrhetate and potassium acetosulfonate; the solvent is a mixture of glycerol and purified water. The content of the preservative in the levetiracetam oral solution is low, the levetiracetam oral solution not only can satisfy a bacteriostasis effect, but also can reduce adverse reactionsand has high safety. The mixture of maltitol type I, ammonium glycyrrhizinate and potassium acetylsulfonate is used as the flavoring agent, so both the effects of flavor correction and thickening areachieved, and the bitterness is reduced. The levetiracetam oral solution contains alanine and / or taurine and other flavor masking agents and has better taste.
Owner:武汉兴华智慧医药科技有限公司
Pharmaceutical composition of acetaminophen and glycyrrhizic acid or salt thereof or derivative thereof
PendingCN106806376ALessen liver damageEasy to useOrganic active ingredientsAntipyreticDiammonium GlycyrrhizinateDISODIUM GLYCYRRHIZATE
The invention relates to a pharmaceutical composition, in particular to a pharmaceutical composition of acetaminophen and glycyrrhizic acid or medicinal salt thereof or medicinal derivative thereof. The weight ratio of acetaminophen to glycyrrhizic acid or medicinal salt thereof or medicinal derivative thereof is (40:1)-(1:1). Glycyrrhizic acid medicinal salt or glycyrrhizic acid medicinal derivative is selected from mono-ammonium glycyrrhizinate, diammonium glycyrrhizinate, monopotassium glycyrrhizinate, dipotassium glycyrrhizinate, disodium glycyrrhizate, trisodium glycyrrhetate, magnesium isoglycyrrhizinate, glycyrrhetinic acid or glycyrrhizin. The invention further relates to an oral drug prepared from the pharmaceutical composition, and the oral drug can be tablets, capsules or particles. By the adoption of the pharmaceutical composition, liver injury caused by acetaminophen can be reduced to a certain extent, using convenience is ensured, and therefore a solution is provided for safer and more efficient use of acetaminophen.
Owner:COSCI MED TECH CO LTD
Fingerprint spectrum construction method and application of Chinese herbal compound containing angelica sinensis
The invention discloses a detection method of a Chinese herbal compound containing angelica sinensis. The method comprises the following steps: taking a Chinese herbal compound test sample and reference substances for detection, wherein the Chinese herbal compound comprises angelica sinensis, cassia twig, licorice, white peony root, ginger and jujube; the reference substances are gallic acid, paeoniflorin, ferulic acid, liquiritin, ammonium glycyrrhizinate and 6-gingerol; and the chromatographic conditions of the detection are as follows: a C18 chromatographic column is adopted, methanol or acetonitrile is used as a mobile phase A, an acid aqueous solution is used as a mobile phase B, and a gradient elution procedure includes that 5%-12% A for 0-20 min is performed; 12%-85% A for 20-90 min is performed; a flow velocity is 0.8-1.2 mL / min; a column temperature is 25-40 DEG C; and a detection wavelength is 210-330 nm; and obtaining component information or component and content information of the Chinese herbal compound according to a detection result. According to the invention, the chemical components of the Chinese herbal compound containing the angelica sinensis are comprehensively and systematically analyzed by applying a high performance liquid chromatography technology, and a theoretical basis is provided for deep research on quality control and a pharmacodynamic material basis.
Owner:HUNAN YINENG BIOLOGICAL PHARMA
Detection method for Guizhishaoyaozhimu decoction
The invention provides a detection method for Guizhishaoyaozhimu decoction. According to the method, high performance liquid chromatography is adopted to detect the Guizhishaoyaozhimu decoction. The method includes the following operation steps of: 1) the preparation of reference solutions: gallic acid, mangiferin, paeoniflorin, prim-O-glucosylcimifugin, liquiritin, 4'-O-beta-glucopyranosyl-5-O-methylvisamminol, cinnamic acid and ammonium glycyrrhizinate reference substances are selected, methanol is added to the above reference substances so as to dissolve the reference substances, and obtained substances are adopted as reference solutions; 2) the preparation of a test solution: Guizhishaoyaozhimu decoction freeze-dried powder is taken, and methanol is added to dissolve the Guizhishaoyaozhimu decoction freeze-dried powder, so that the test solution can be obtained; and 3) detection: the reference solutions and the test solution are separately injected into a liquid chromatograph. Themethod of the invention can be used to not only detect the gallic acid, mangiferin, paeoniflorin, prim-O-glucosylcimifugin, liquiritin, 4'-O-beta-glucopyranosyl-5-O-methylvisamminol, cinnamic acid andammonium glycyrrhizinate, but also realize the overall quality control of the Guizhishaoyaozhimu decoction. The detection method can provide a methodological basis for research on the material basisof the Guizhishaoyaozhimu decoction and has a popularization and application value.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE +1
Preparation method of glycyrrhizic acid
The invention discloses a preparation method of glycyrrhizic acid. The preparation method comprises the following steps: firstly, adding glycyrrhizic acid crude powder into an ethanol solution for extracting, adding an extracting solution into an ammoniating solution, stirring, crystallizing and filtering; secondly, carrying out ion exchange on an obtained mono-ammonium glycyrrhizinate crude product in strong acidic cation resin to obtain a glycyrrhizic acid solution, adding the ammoniating solution into the glycyrrhizic acid solution, and stirring, crystallizing and filtering to obtain mono-ammonium glycyrrhizinate; thirdly, adding water into the mono-ammonium glycyrrhizinate for dissolving, and acidizing and filtering to obtain a glycyrrhizic acid solid; fourthly, dissolving the glycyrrhizic acid solid with an alkaline solution, diluting, absorbing with macroporous adsorption resin, eluting and collecting eluant; fifthly, transforming the eluant by using strong acid cation exchange resin, and removing a solvent of a transformation solution to obtain glycyrrhizic acid powder. The preparation method disclosed by the invention has the advantages of short process period, low cost and high safety; the obtained product has the characteristics of high yield, good color and high purity. The glycyrrhizic acid prepared by the preparation method disclosed by the invention can be applied to the fields of medicine and food.
Owner:JIANGSU TIANSHENG PHARMA
Production process for jointly separating high-purity liquiritin, bitterness-removed glycyrrhizin and glycyrrhiza uralensis fisch total flavonoids from mono-ammonium glycyrrhizinate mother liquor paste
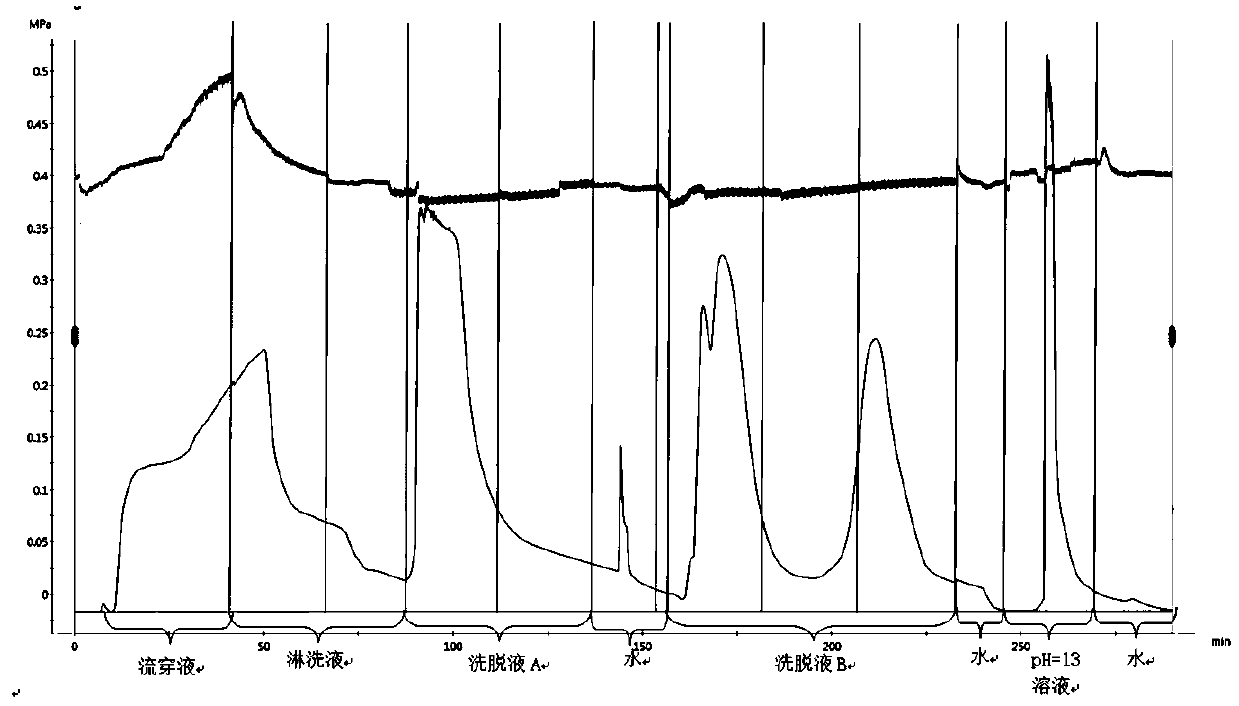
The invention discloses a production process for jointly separating high-purity liquiritin, bitterness-removed glycyrrhizin and glycyrrhiza uralensis fisch total flavonoids from mono-ammonium glycyrrhizinate mother liquor paste. The production process comprises the following steps that (1) the mono-ammonium glycyrrhizinate mother liquor paste is taken, water is added to adjust the pH value, the mono-ammonium glycyrrhizinate mother liquor paste is dissolved and passes through a resin column 1# to be subjected to chromatography, water elution is carried out to obtain eluent for later use, a resin column 2# is subjected to elution through gradient dilute ethanol, and after the eluent is concentrated and dried under reduced pressure, a liquiritin crude product is obtained; ethanol with the concentration of 95% is added into the crude product, and recrystallization, filtration and drying are carried out to obtain the high-purity liquiritin; (2) the eluent for later use is taken, flows through a resin column 3# and is subjected to water elution, and the eluent is concentrated and dried to obtain the bitterness-removed glycyrrhizin; and (3) the resin columns 1#, 2# and 3# are regeneratedby using the ethanol with the concentration of 95%, eluent is collected and combined, and concentration and drying are carried out to obtain the glycyrrhiza uralensis fisch total flavonoids. Accordingto the production process for jointly separating the high-purity liquiritin, the bitterness-removed glycyrrhizin and the glycyrrhiza uralensis fisch total flavonoids from the mono-ammonium glycyrrhizinate mother liquor paste, the mother liquor paste after production of mono-ammonium glycyrrhizinate is adopted as a raw material, and the three products are separated and purified in a combined mode,so that the comprehensive utilization degree of the raw material is improved, the product value is high, cost is low, reproducibility is good, and the production process is suitable for industrial large-scale production.
Owner:高颖
Method for capturing and separating effective components in liquorice by utilizing mixed-mode agarose gel medium
ActiveCN111001189AExtend engagement and criticalityWide operating conditionsIon-exchange process apparatusIon-exchanger regenerationNatural productIon exchange
The invention relates to a method for capturing and separating effective components in liquorice by utilizing a mixed-mode agarose gel medium. The method is characterized in that the mixed-mode agarose gel medium with ion exchange and hydrophobic ligands is used for capturing and separating acidic saponin substances and flavonoid substances in liquorice; the two substances are effectively adsorbedin the agarose gel medium in an adsorption mode, and effective separation of the acidic saponin substances and the flavonoid substances is realized by changing the pH value of an eluent. According tothe method for capturing and separating liquiritin and glycyrrhizic acid substances in liquorice, the result can approximately reach that the purity of the liquiritin substances is 65%-95%, and the yield is 80%-98% (calculated by taking the liquiritin as a standard substance); the purity of glycyrrhizic acid substances is 60-95%, the yield is 75-95% (calculated by taking ammonium glycyrrhizinateas a standard substance), the purity and the yield are relatively high, and the method has important significance for a component separation process of natural products and a traditional Chinese medicine extraction and purification process.
Owner:TAIZHOU GUOKEHUAWU BIOMEDICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com