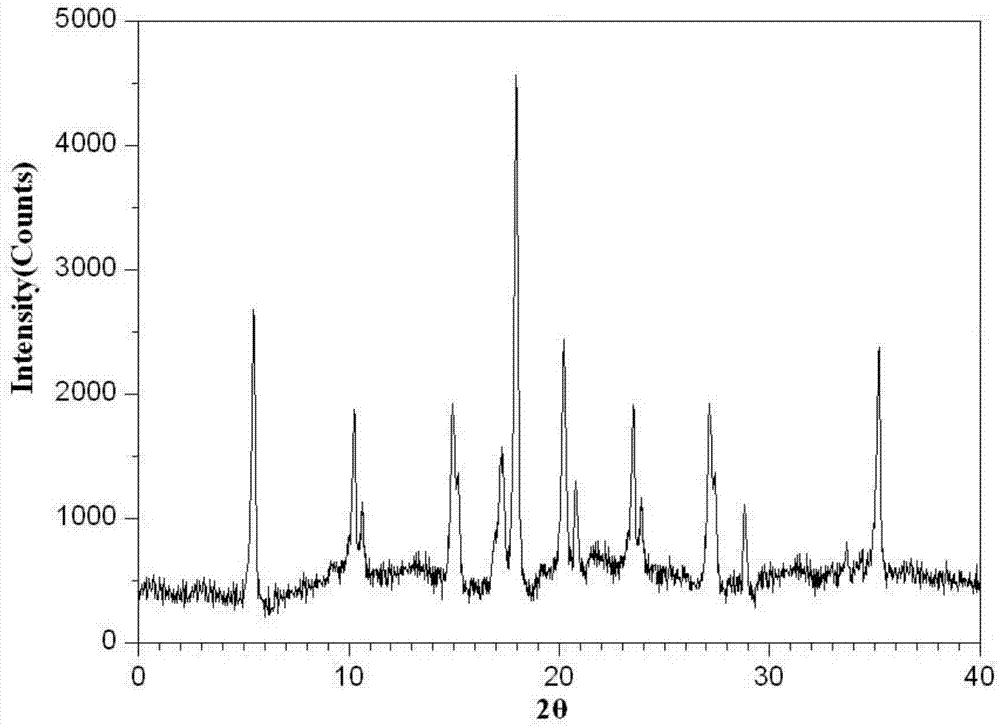
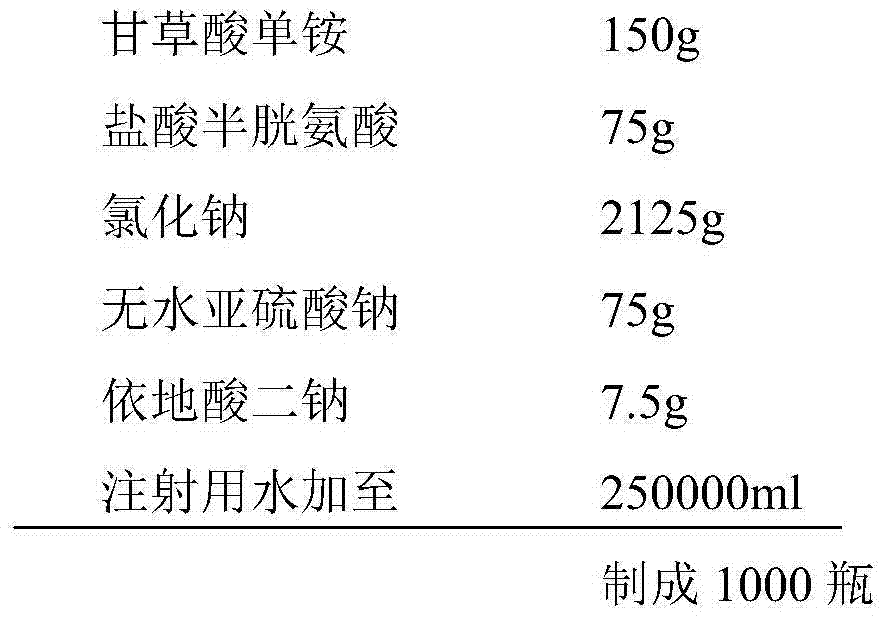
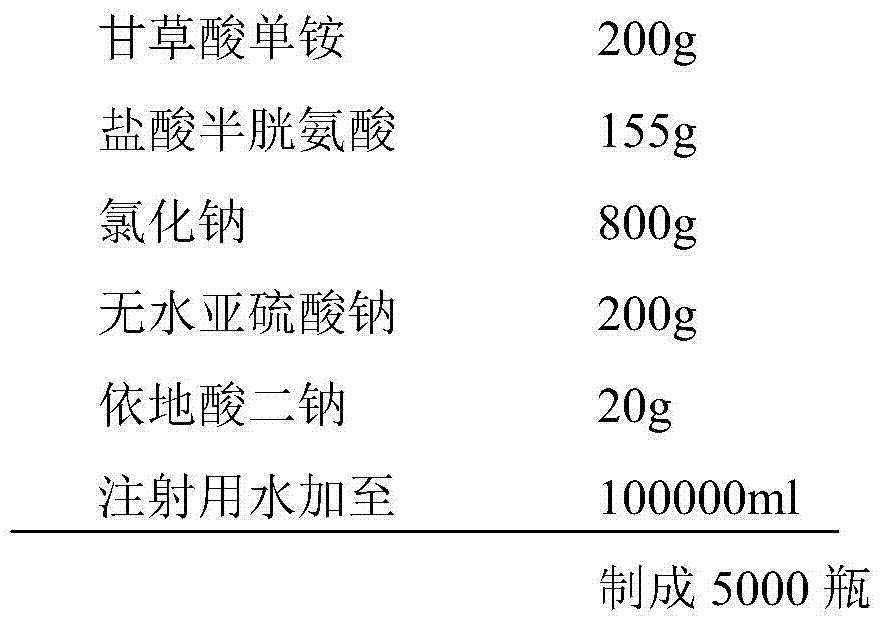
Ammonium glycyrrhizinate compound and pharmaceutical composition containing ammonium glycyrrhizinate
A monoammonium glycyrrhizinate compound technology, applied in the field of medicine, can solve the problems of poor solubility in hot water and poor water solubility, and achieve the effect of good resolubility, high solubility and easy dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, the preparation of monoammonium glycyrrhizinate compound
[0040] Take monoammonium glycyrrhizinate (C 42 h 65 NO 16 2H 2O) 50g of raw material drug, add N,N-dimethylformamide / ethanol mixed solvent, wherein the ratio of monoammonium glycyrrhizinate raw material drug to mixed solvent is 1g:6ml, N,N-dimethylformamide in the mixed solvent The volume ratio of formamide to ethanol is 1:3, then add 0.10g of activated carbon, stir for adsorption, filter for decarbonization and sterilization to obtain a clear solution. Reflux at 45°C for 2 hours, cool down to 30°C, keep warm, and add chloroform dropwise at a stirring rate of 25r / min. The volume ratio of the chloroform to the mixed solvent is 3:1. After the dropwise addition, continue to keep warm and stir After 20 min, the stirring was stopped, the temperature was lowered to 0°C, and the mixture was allowed to stand at this temperature for 2 hours, filtered, washed with ethanol three times, and dried under red...
Embodiment 2
[0042] Embodiment 2, the preparation of monoammonium glycyrrhizinate compound
[0043] Take monoammonium glycyrrhizinate (C 42 h 65 NO 16 2H 2 O) 50g of raw material drug, add N,N-dimethylformamide / ethanol mixed solvent, wherein the ratio of monoammonium glycyrrhizinate raw material drug to mixed solvent is 1g:10ml, N,N-dimethylformamide in the mixed solvent The volume ratio of formamide to ethanol is 1:5, then add 0.10 g of activated carbon, stir for adsorption, filter for decarbonization and sterilization, and obtain a clear solution. Reflux at 50°C for 3 hours, cool down to 40°C, keep warm, and add chloroform dropwise at a stirring rate of 30r / min, the volume ratio of chloroform to mixed solvent is 7:1, continue to keep warm and stir after the dropwise addition After 30 minutes, the stirring was stopped, the temperature was lowered to 5°C, and the mixture was allowed to stand at this temperature for 6 hours, filtered, washed with ethanol three times, and dried under red...
Embodiment 3
[0045] Embodiment 3, the preparation of monoammonium glycyrrhizinate compound
[0046] Take monoammonium glycyrrhizinate (C 42 h 65 NO 16 2H 2 O) 50g of raw material medicine, add N,N-dimethylformamide / ethanol mixed solvent, wherein the dosage ratio of monoammonium glycyrrhizinate raw material medicine and mixed solvent is 1g:8ml, N,N-dimethylformamide in mixed solvent The volume ratio of formamide to ethanol is 1:4, then add 0.10g of activated carbon, stir for adsorption, and filter to decarbonize and sterilize to obtain a clear solution. Reflux at 47°C for 2.5h, cool down to 34°C, keep warm, and add chloroform dropwise at a stirring rate of 28r / min. The volume ratio of chloroform to mixed solvent is 5:1, and keep warm after the dropwise addition Stir for 25 minutes, then stop stirring, cool down to 2°C, let stand at this temperature for 3 hours, filter, wash with ethanol three times, and dry under reduced pressure for 3 hours to obtain the obtained product.
[0047] The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com