Method for controlling quality of Longdanxiegan Capsule
A quality control method, a technology for gentian purging the liver, applied in the directions of measuring devices, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems such as unfavorable control, no detection of geniposide content, cumbersome detection process, etc. Easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] Prescription Gentian 120g, Bupleurum 120g, Scutellaria baicalensis 60g, Gardenia (fried) 60g, Alisma 120g, Akebia 60g, Plantago seed (salt fried) 60g, Angelica (wine fried) 60g, Rehmannia glutinosa 120g, licorice (honey-roasted) )60g
[0021] The above ten flavors are decocted twice with water, 1.5 hours each time, filtered, and the filtrates are combined, concentrated to a relative density of 1.25-1.28 (80°C), added ethanol to make the alcohol content reach 70%, and allowed to stand for 24 hours Above, filter, recover ethanol from the filtrate, add appropriate amount of dextrin, adjust to a relative density of about 1.18 (80°C), spray dry, and make extract powder. Take 1 part of extract powder, 1.5 parts of auxiliary materials (including the amount of dextrin mentioned above), mix well, make granules, dry at low temperature, pack into capsules, and make 1000 granules.
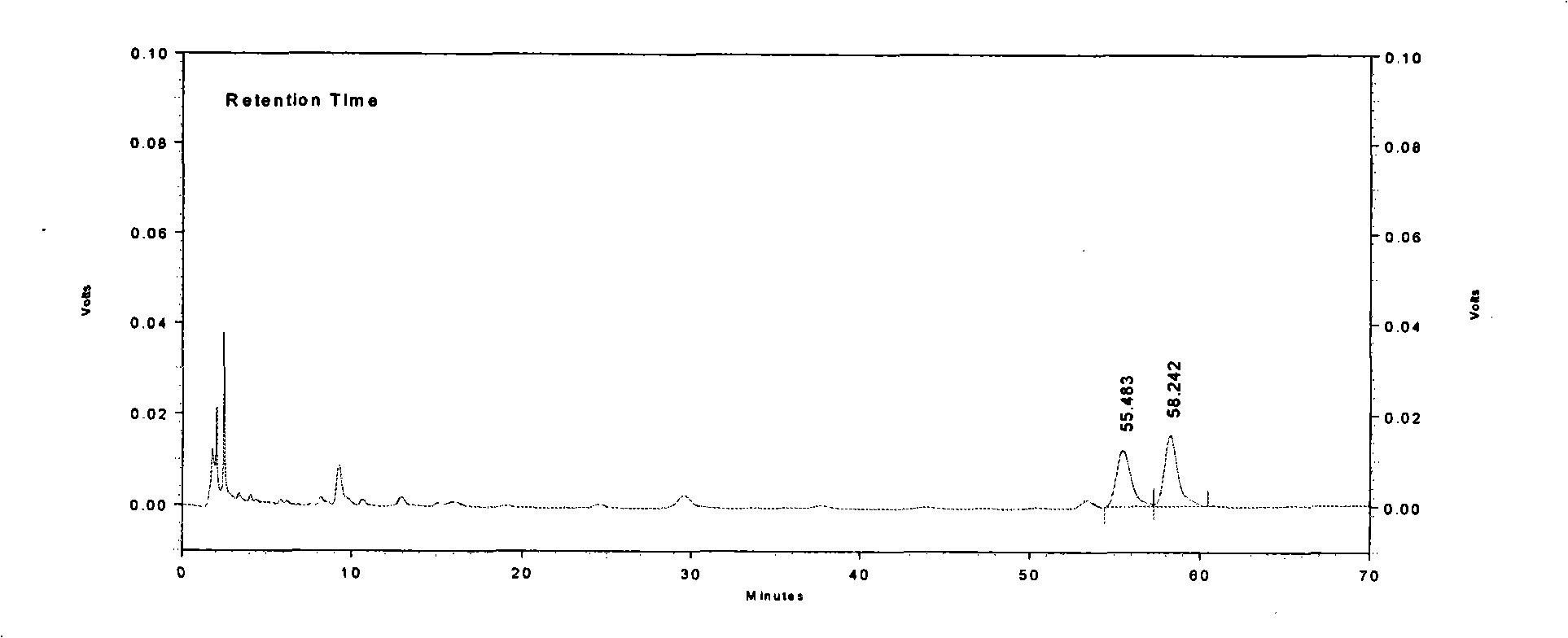
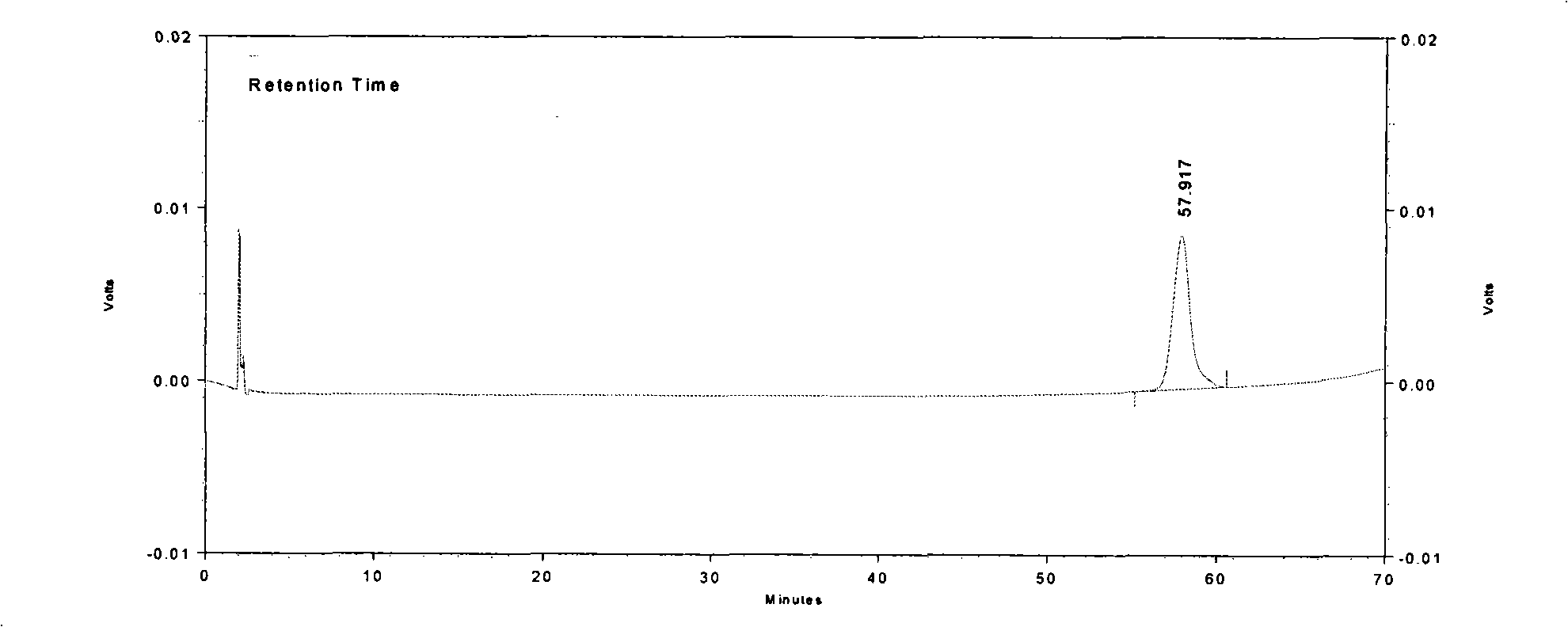
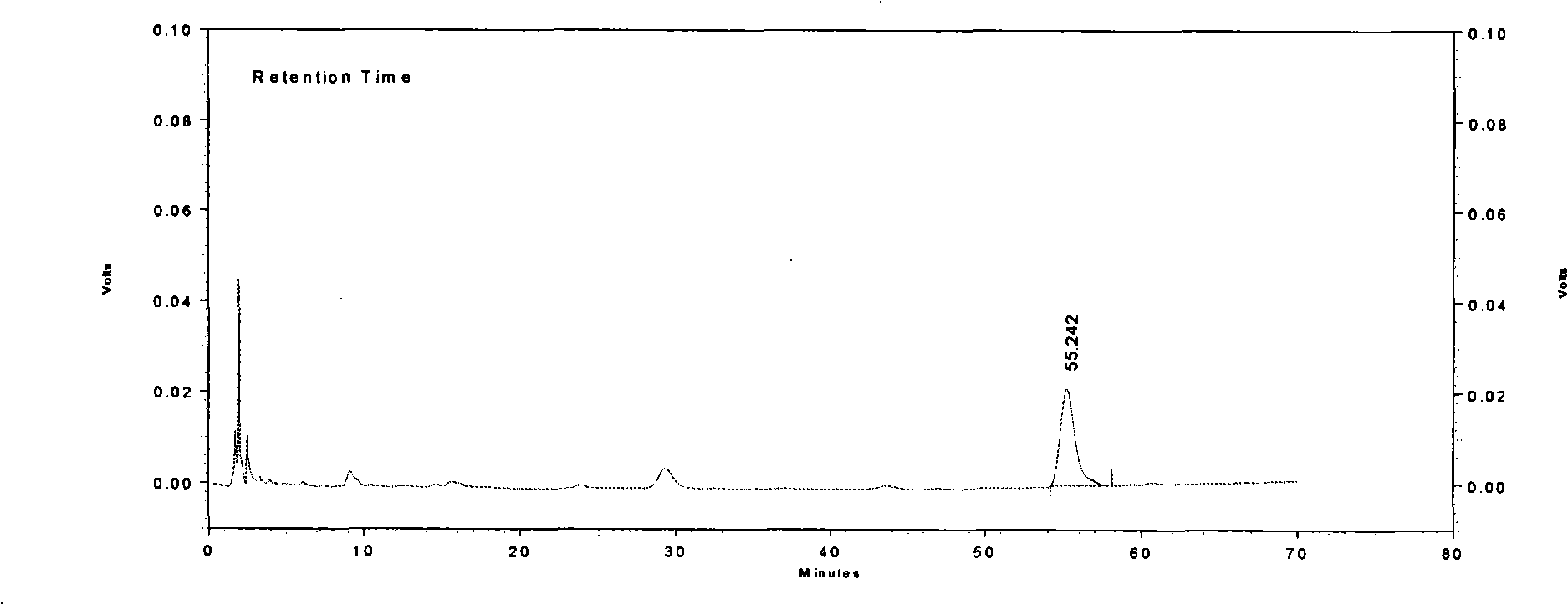
[0022] Character identification methods are: the content of Longdan Xiegan Capsules is brownish-yel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com