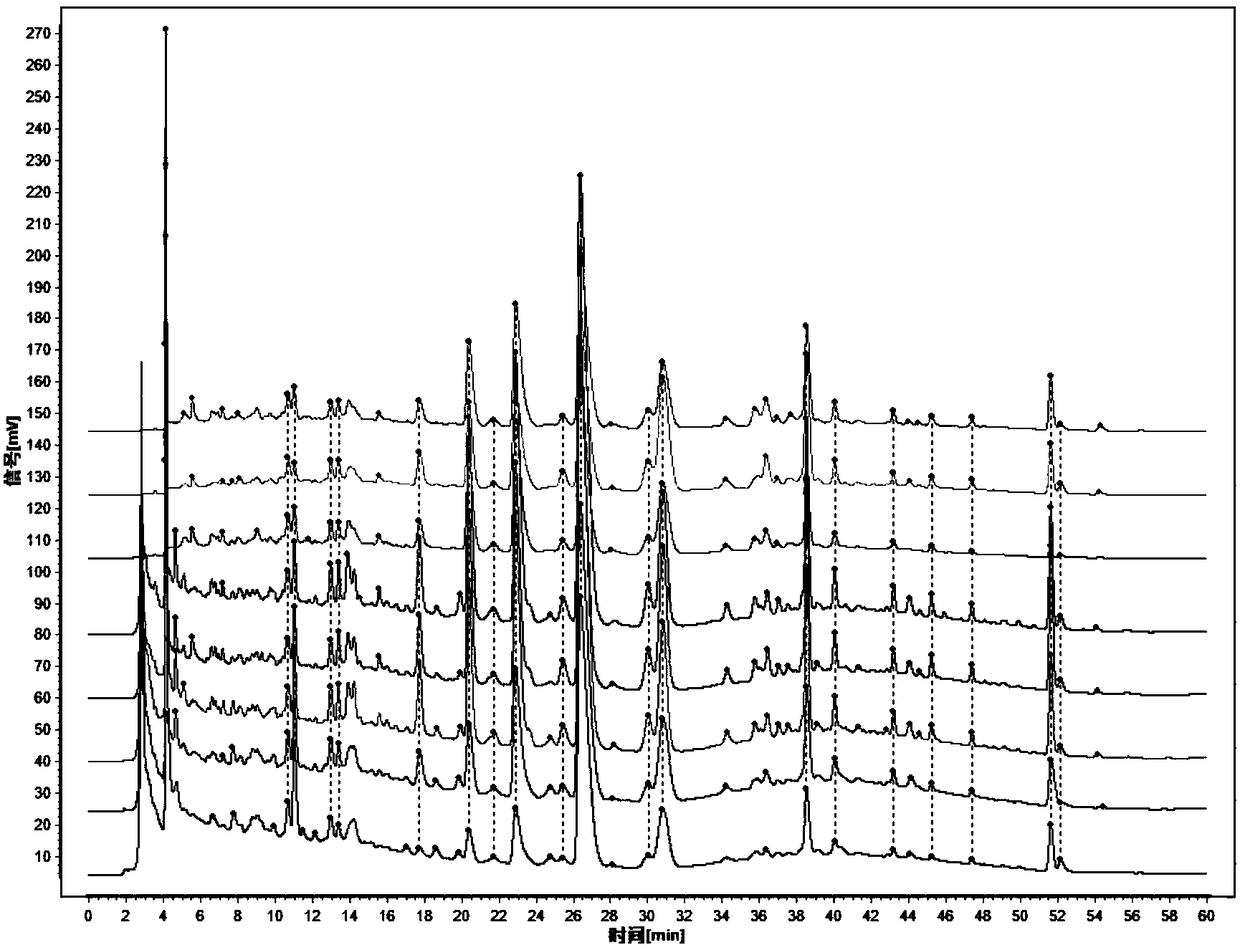
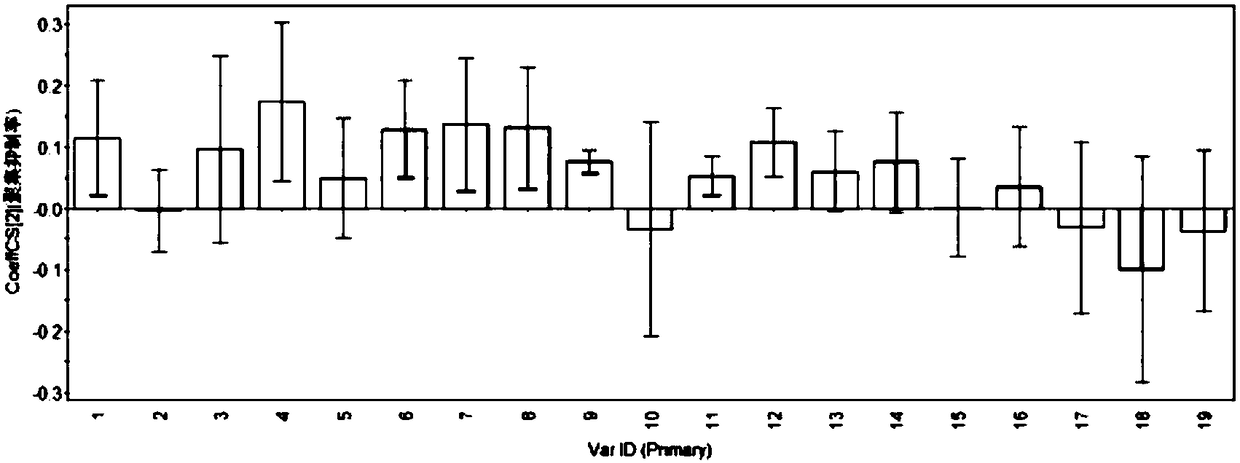
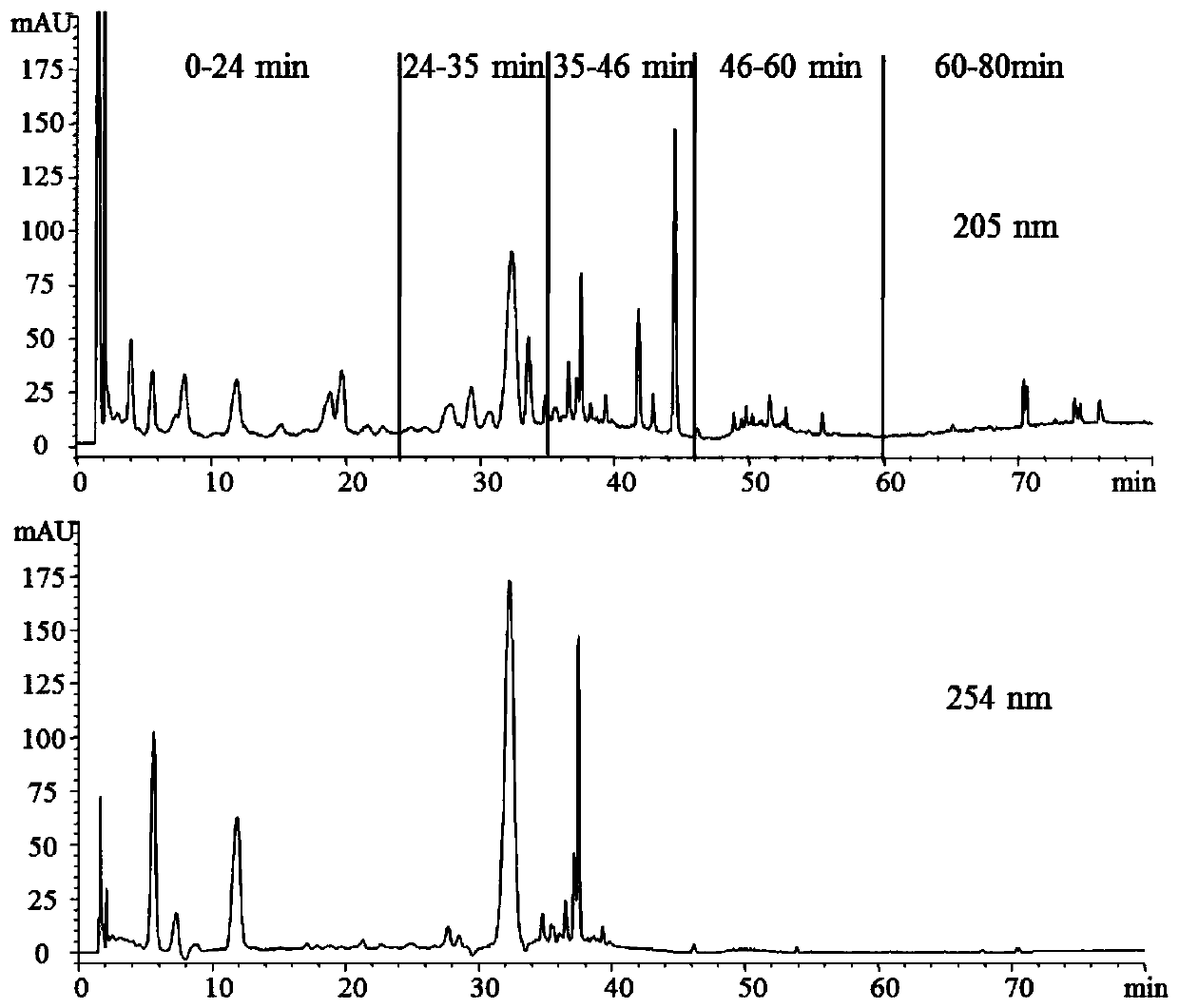
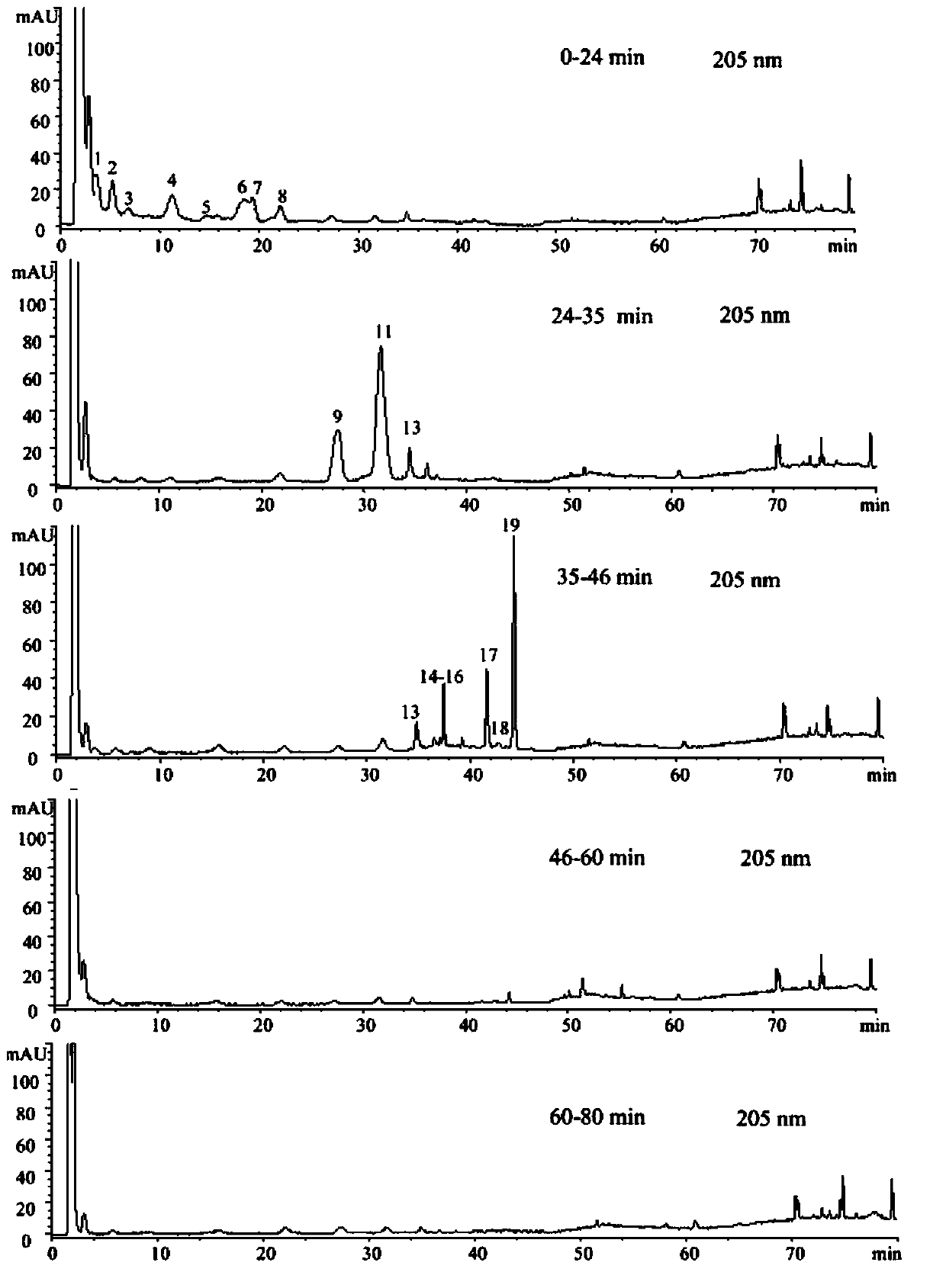
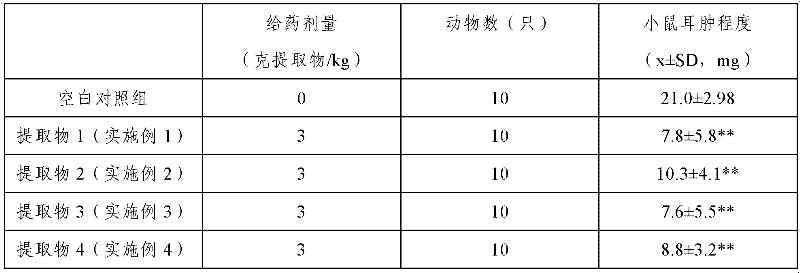
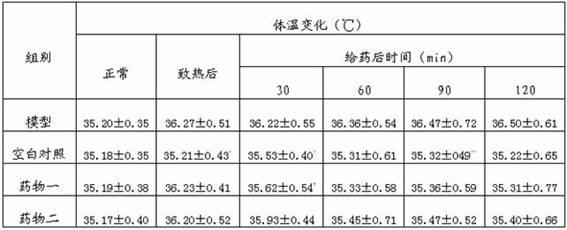
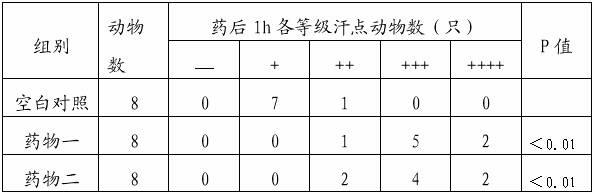
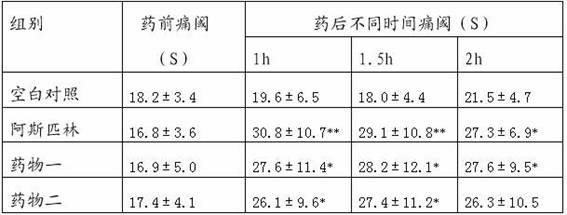
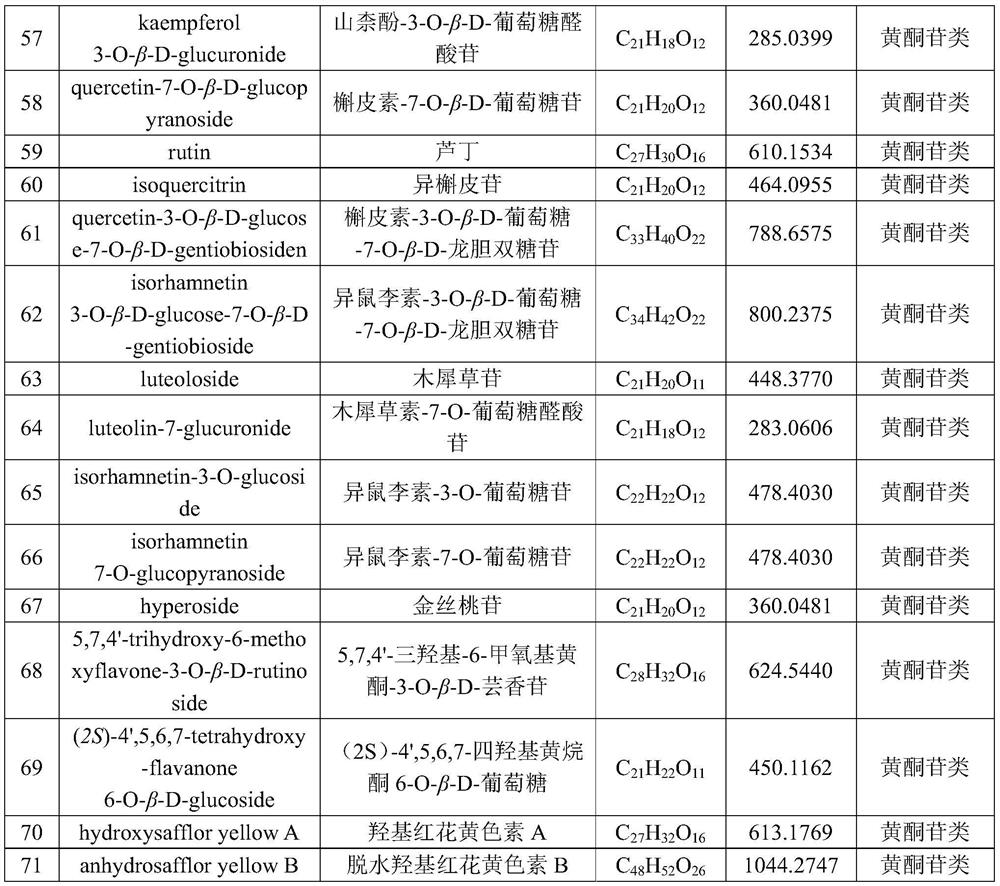
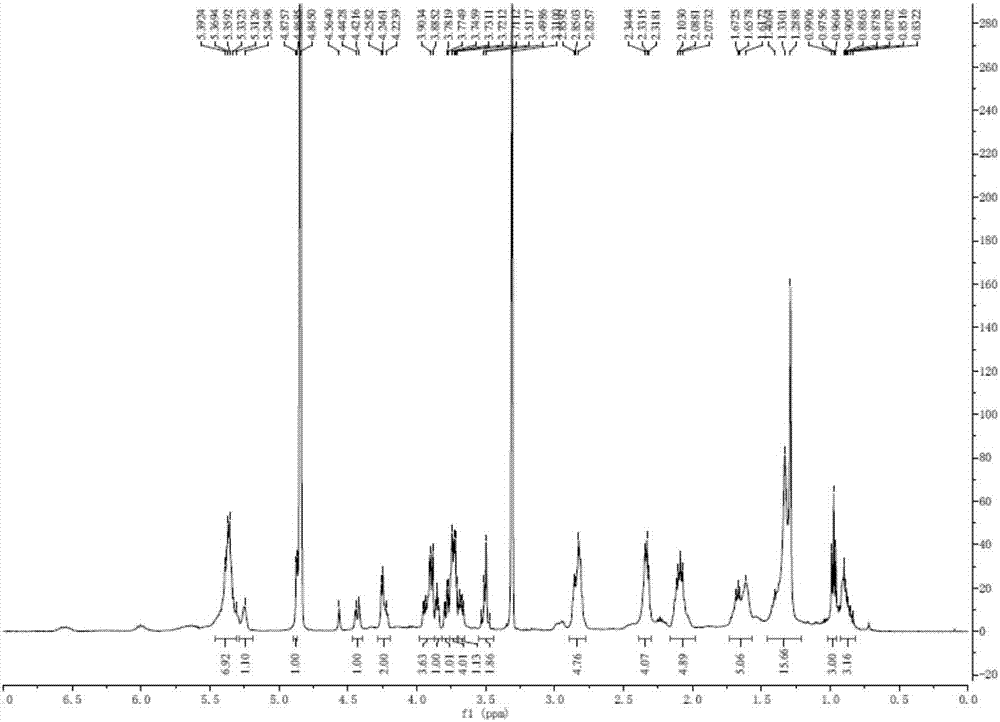
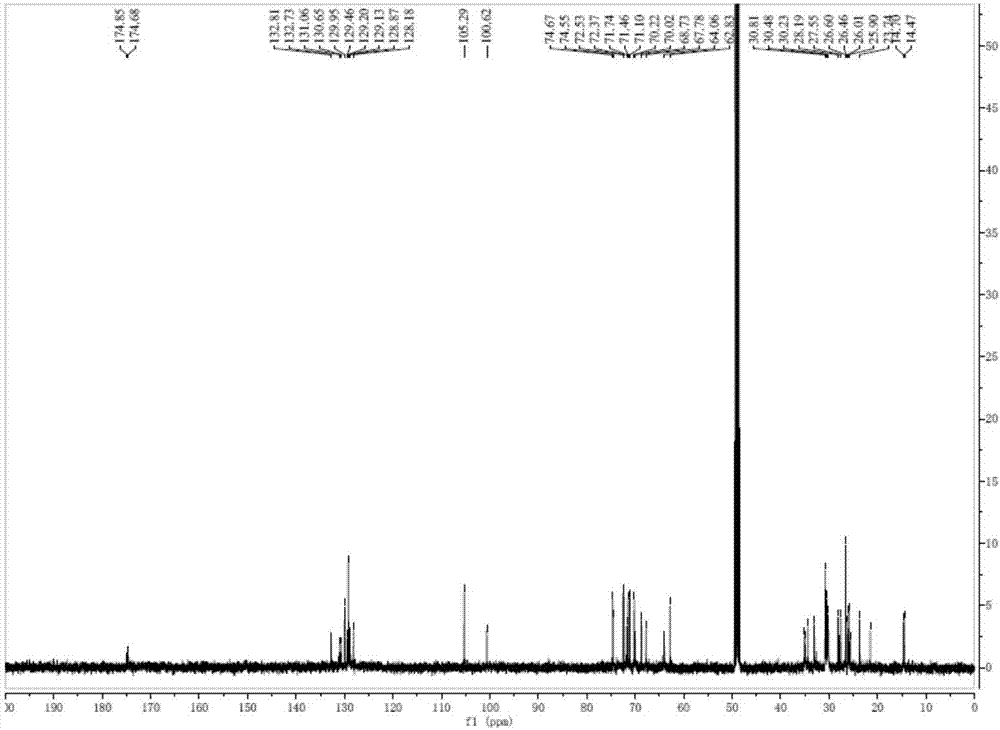
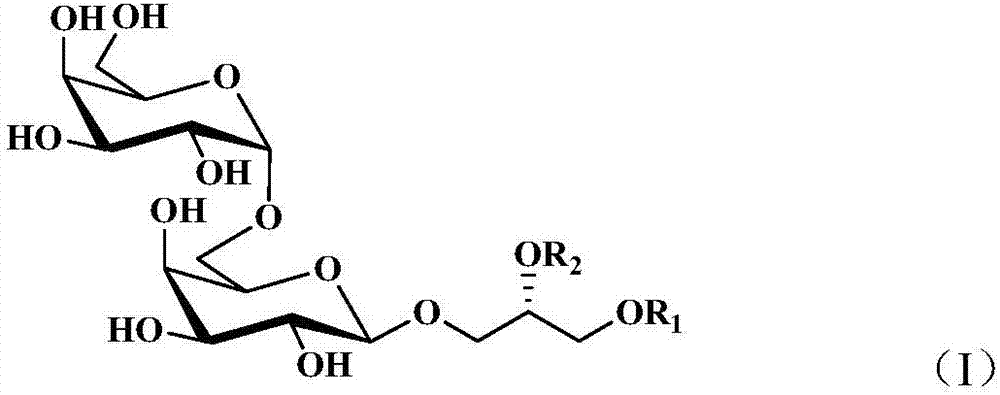
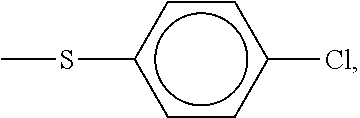
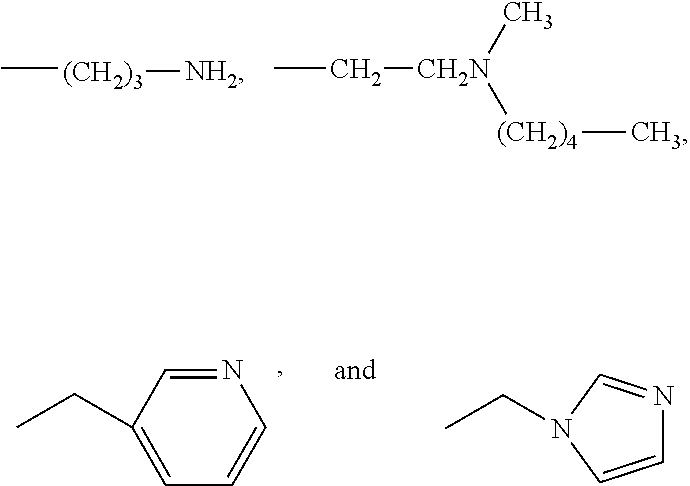
Patents
Literature
127 results about "Pharmacological Substance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmacology is the study of how substances interact with living organisms to produce a change in function. If substances have medicinal properties, they are considered pharmaceuticals. The field encompasses drug composition and properties, interactions, toxicology, therapy, and medical applications and antipathogenic capabilities.
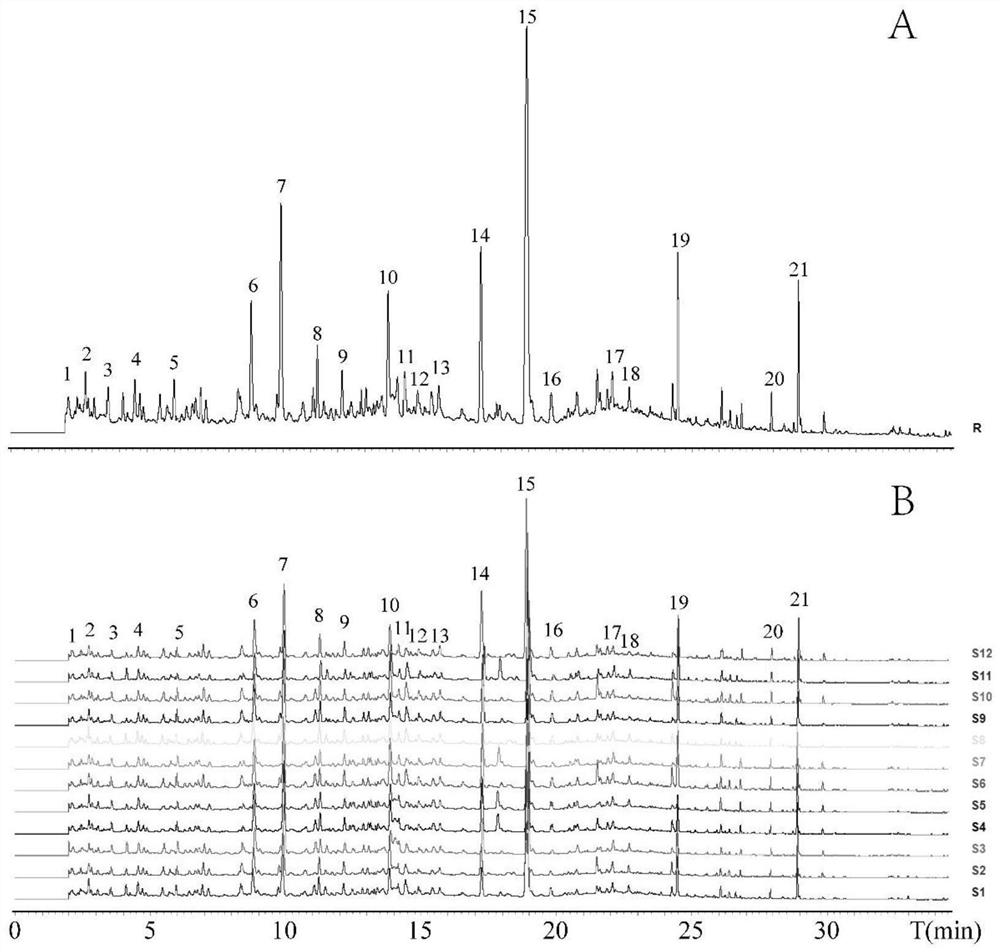
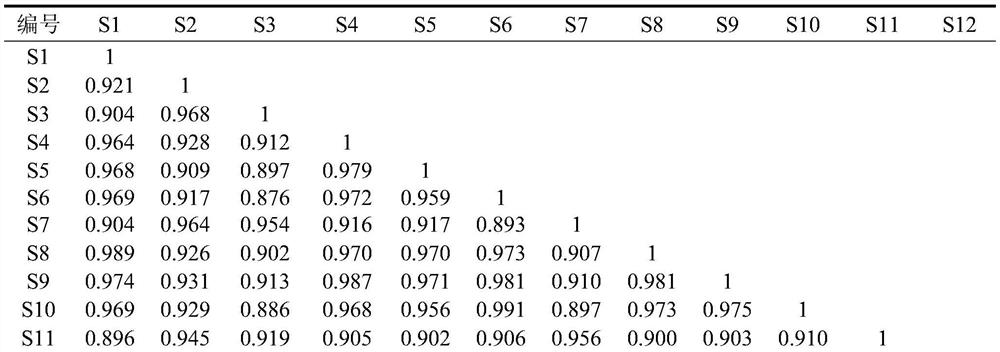
Quality control method of liuwei wuling tablets
InactiveCN105758962AReduce wear and tearShort analysis timeComponent separationBiological testingMedicinal herbsHplc fingerprint
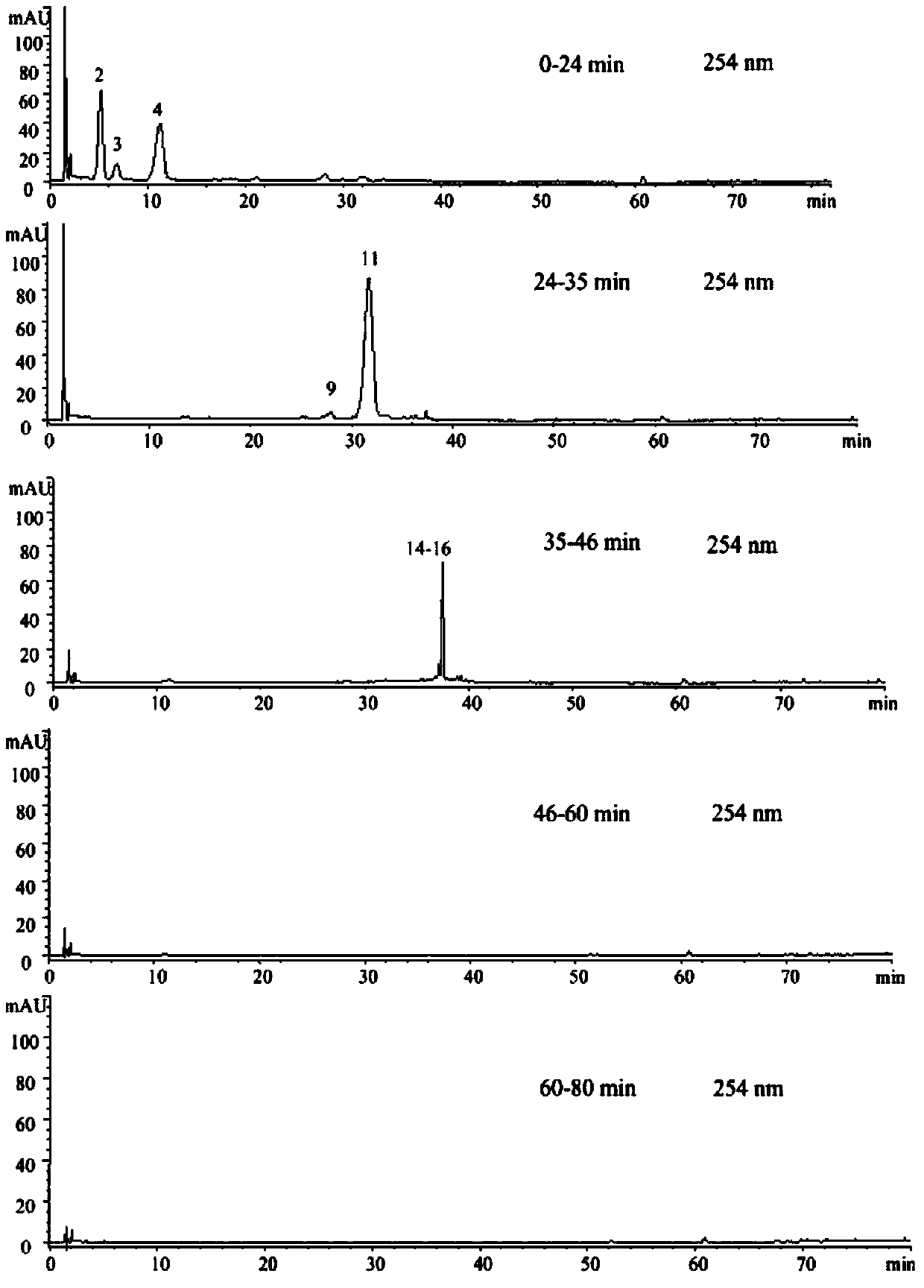
The invention relates to the technical field of compound traditional Chinese preparations, and in particular relates to a quality control method of liuwei wuling tablets. The quality control method is characterized in that 20 different proportion groups are formed by randomly sampling six medicinal materials of the liuwei wuling tablets; a corresponding HPLC fingerprint spectrum is established, and in-vitro anti-hepatic fibrosis activity is measured; and an SPSS software is utilized to analyze, so that a pectrum-effect relationship of in-vitro anti-hepatic fibrosis effect of the liuwei wuling tablets is established. The relatively perfect quality control method is established on the basis of pharmacodynamic substances of the liuwei wuling tablets. Compared with a conventional single-index content measuring method, the quality control method is more comprehensive, scientific and standard, and has the advantages that the content of multiple components is simultaneously measured, so that not only the analysis time is shortened, but also the loss of a solvent is saved.
Owner:山东世博金都药业有限公司
Method for evaluating chemical composition of Rosa xanthina on basis of antithrombotic spectrum-effect relationship
ActiveCN108195989AComprehensive and accurate spectrum effect basisClear chemical compositionComponent separationMathematical modelSeparation technology
The invention discloses a method for evaluating chemical composition of Rosa xanthina on the basis of antithrombotic spectrum-effect relationship. The method comprises the following steps: preparing extract of different polar components of the Rosa xanthina with a modern separation technology; establishing fingerprint of extract of each component with high-performance liquid chromatography, and calibrating characteristic peaks; evaluating antithrombotic activity of different extract on the basis of platelet aggregation inhibition rate, prothrombin time, thrombin time and activated partial thromboplastin time as indexes; substituting fingerprint characteristic peak data and pharmacodynamical activity data into a mathematical model for spectrum-effect correlation analysis, and evaluating pharmacodynamical activity of the characteristic peaks. With adoption of the method for evaluating the chemical composition of the Rosa xanthina on the basis of the antithrombotic spectrum-effect relationship, the antithrombotic chemical composition in the Rosa xanthina can be evaluated rapidly and accurately, a scientific and effective method is provided for research of pharmacodynamic material basis and quality control of the Rosa xanthina, and reference is provided for further development of Rosa xanthina drugs or health care products for treating thrombotic diseases.
Owner:山西省医药与生命科学研究院
Novel method for screening traditional Chinese medicine anti-osteoporosis active component with zebra fish osteoporosis model
InactiveCN103424362ATime consumingLabor intensiveMaterial analysis by optical meansBiological testingSolventGlucocorticoid
The invention discloses an improved model organism zebra fish osteoporosis model application method and application of the improved model organism zebra fish osteoporosis model application method in screening the traditional Chinese medicine anti-osteoporosis active component by uniting with the modern chromatography technology. According to the improved model organism zebra fish osteoporosis model application method, the features of high-efficiency separation and analysis of traditional Chinese components of the modern chromatography technology and the united technology of the modern chromatography technology are combined, a target component or component groups are captured or knocked out or authenticated directionally. The component or the component groups which are captured or knocked out are screened in an anti-osteoporosis active mode with the zebra fish osteoporosis model. The traditional Chinese components are separated and analyzed according to the optimized chromatography and mass spectrum conditions. The traditional Chinese components are analyzed according to features of ultraviolet ray absorption, molecular weight and the like. The target component or the component groups are flexibly determined as required, the target component or the component groups of different preserving time periods are collected and thus, the captured or knocked-out traditional Chinese medicine component or the component groups are obtained. After an organic solvent is removed, a proper solvent is used for carrying out dissolution. The zebra fish osteoporosis model which carries out inducing with glucocorticoids rapidly evaluates anti-osteoporosis activity of the traditional Chinese component or the component groups. The modern chromatography and the united technology of the modern chromatography overcome the defects that a traditional chemical component is low in separation speed and a large number of trace components are hard to obtain. The zebra fish osteoporosis model breaks through the bottleneck that the trace components are hard to undergo anti-osteoporosis activity high-efficiency evaluation. The modern chromatography and the united technology are united in an organic mode so as to break through the restraint of an evaluation model and the number of chemical compounds. The anti-osteoporosis pharmacodynamic material basis is provided for screening the traditional Chinese medicine efficiently.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
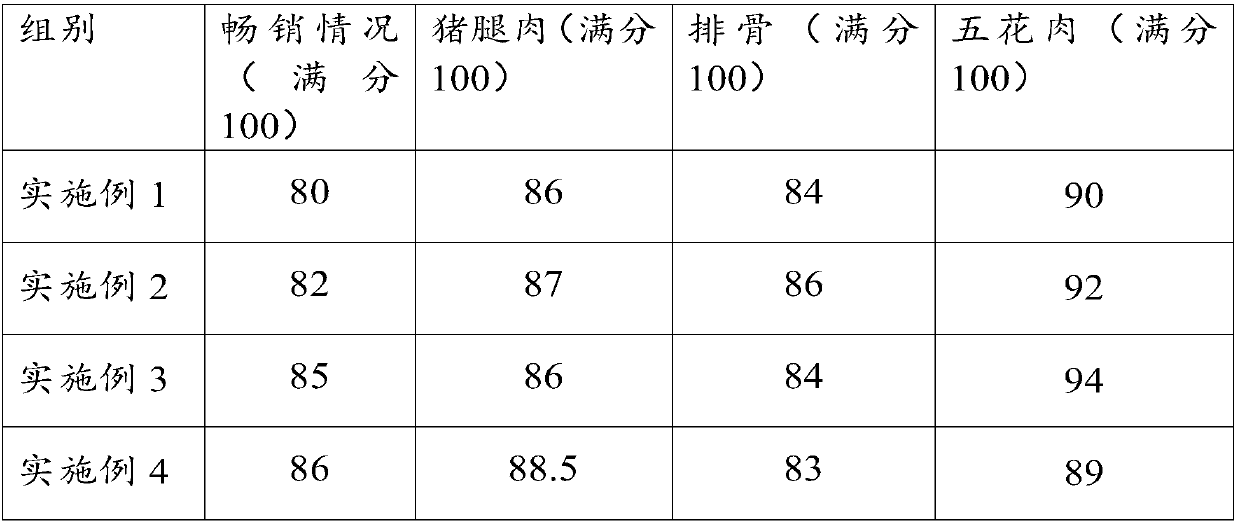
Pig feed capable of improving meat quality, and preparation method thereof
InactiveCN107712363AEnhance physical fitnessIncrease appetiteFood processingAnimal feeding stuffBiotechnologyGlycerol
The invention relates to the field of pig feed, in particular to pig feed capable of improving the meat quality, and a preparation method thereof. The pig feed capable of improving the meat quality mainly comprises the following raw materials: corns, bean pulp, oat, fish meal, rapeseeds, fructus forsythiae, honeysuckle flowers, cyrtomium fortune, radices sileris, radix bupleuri, astragalus membranaceus, bighead atractylodes rhizome, poria cocos, dried orange peel, Chinese angelica, white paeony root, dandelion, motherwort, purslane, codonopsis pilosula and feed additives. The feed additives comprise glycerol monolaurate, calcium propionate, amino acid and sodium molybdate. The pig feed capable of improving the meat quality, which is provided by the embodiment of the invention, has multiplefunctions of reinforcing the vital essence and strengthening the primordial qi, regulating physiological function, improving immunity, resisting inflammation, resisting bacteria, resisting viruses and the like through the proportioning of nutritional substances, pharmacological substances, amino acid and various cereals, increases absorption and utilization of nutritional components by pigs and enables the meat to be delicious.
Owner:GUIZHOU DAXING AGRI SCI & TECH DEV CO LTD
Coated suture thread and production thereof
InactiveUS20090177228A1Easily be used for building matrixReduce decreaseSuture equipmentsPretreated surfacesFiberDamages tissue
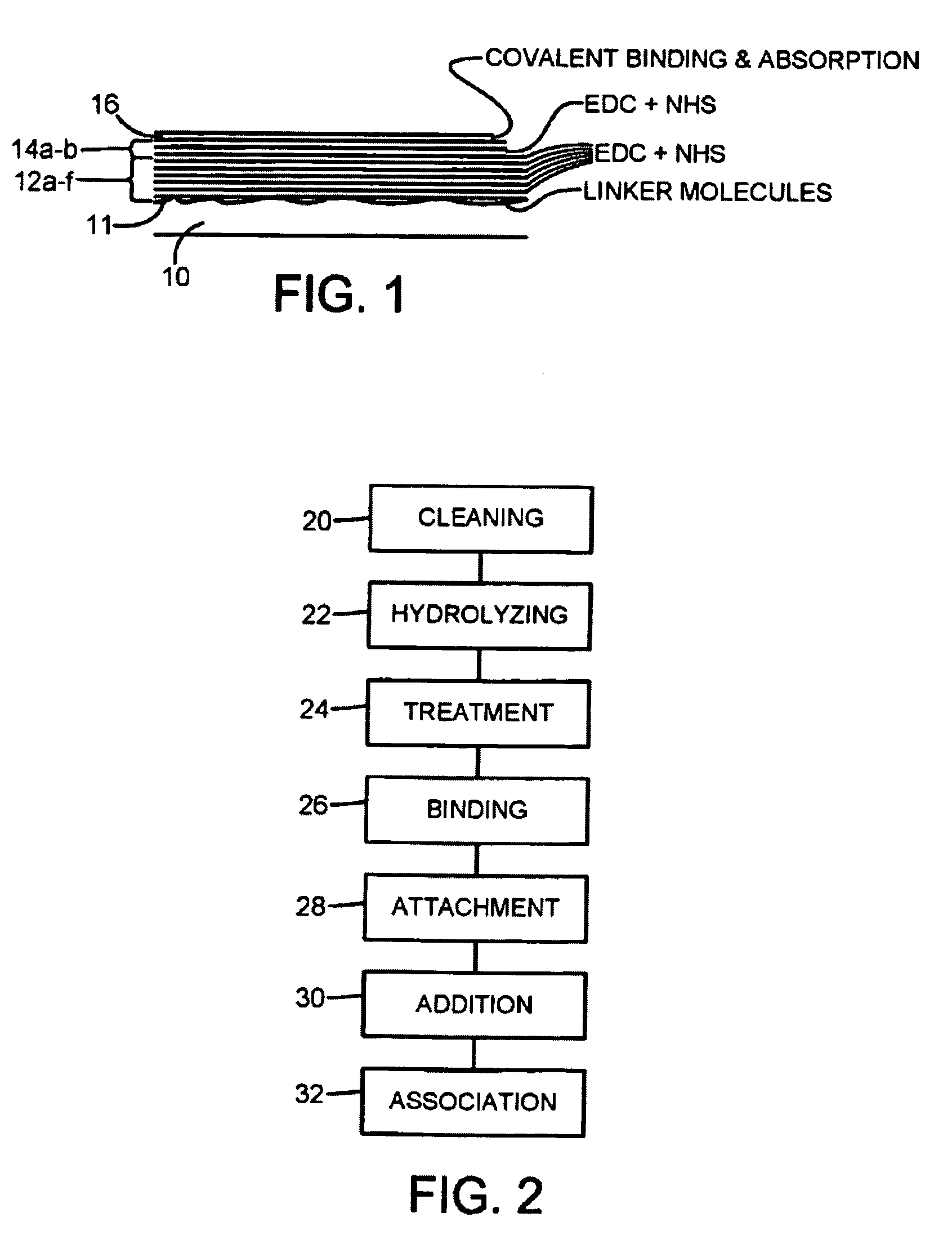
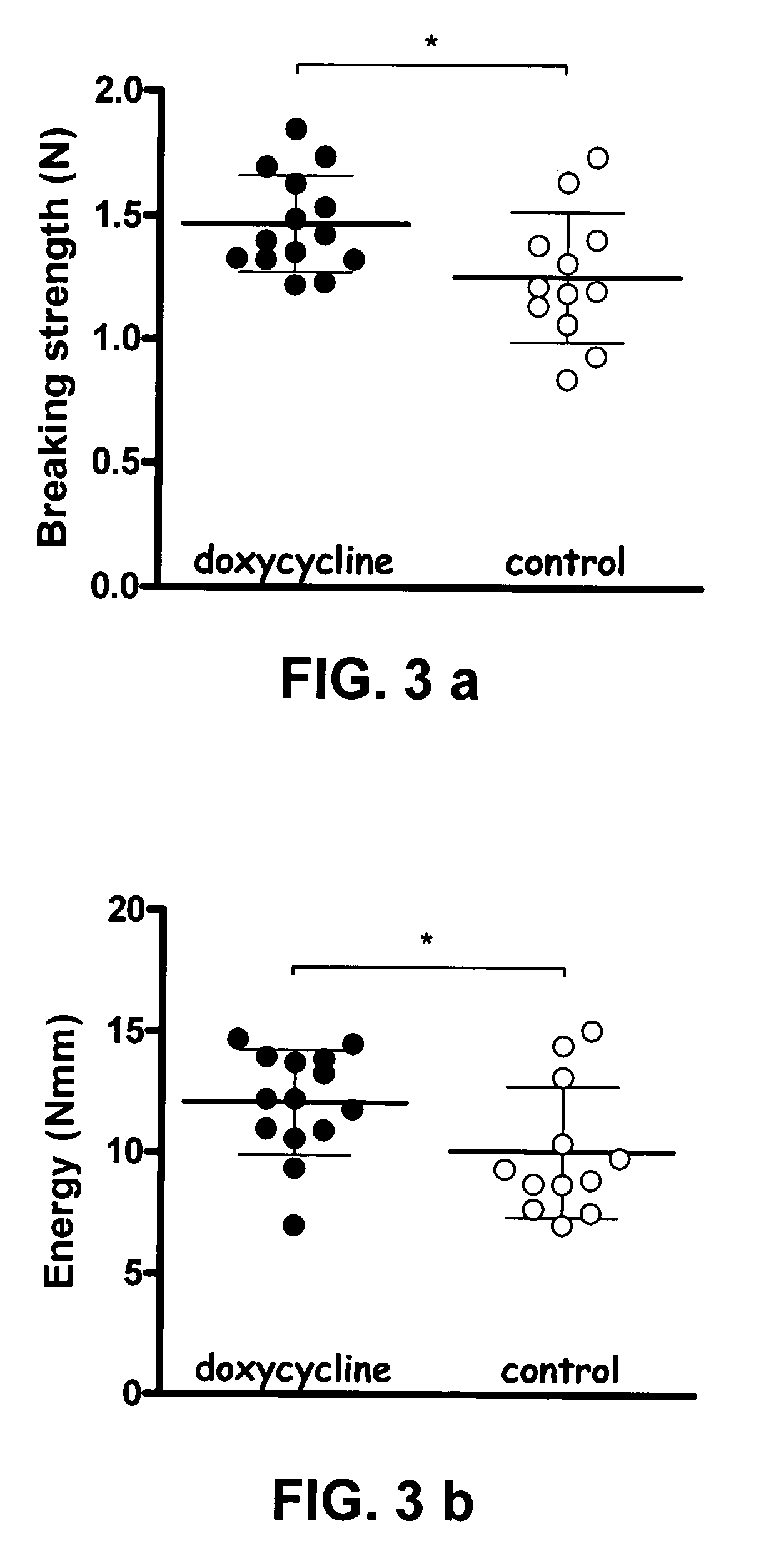
Disclosed is a coated suture thread comprising a matrix formed by an immobilized and crosslinked plurality of fibrinogen layers into and / or onto which one or several pharmacological substances that inhibit tissue break-down, such as MMP inhibitors and / or corticosteroids and / or COX inhibitors, are attached and / or associated. Further, a method of producing such a coated suture thread as well as the use thereof for suturing damaged tissue, such as damaged tendon, ligament, intestine and / or skin, are described.
Owner:ADDBIO
R language-based optimal extraction technology for extracting saponin and total flavonoids in liquorice
InactiveCN106511448ASimple extraction processGood extraction processBiological neural network modelsGenetic algorithmsMathematical modelEntropy weight method
The invention discloses an R language-based optimal extraction technology for extracting saponin and total flavonoids in liquorice. A central composite design experiment in a response surface method is adopted, the saponin content and flavone content in liquorice are used as detection indexes, and an entropy weight method in an R language environment is adopted for weight assignment of the two indexes, a BP neural network and genetic algorithm mathematical model is established, and target optimizing is performed for the extraction technology to obtain an optimal extraction technology. The optimal extraction conditions are as follows: the ammonia concentration is 0.62%, the ethanol concentration is 64%, the reflux time is 1.8h, and the liquid-solid ratio is 12:1. Relative error between the comprehensive evaluation predicted value of the model and the average comprehensive evaluation value of a verification experiment is 1.43%, proving relatively good predictability of the neutral network and genetic algorithm. In the invention, the established mathematical model searching for the optimal extraction conditions for extracting saponin and total flavonoids in liquorice is scientific and feasible, and new reference and idea are provided for optimizing multiple targets of the chemical components and even effective substance basis of traditional Chinese medicine.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
Pharmacodynamic material basis screening method of artemisia capillaries soup
The invention discloses a pharmacodynamic material basis screening method of artemisia capillaries soup, relates to a pharmacodynamic material basis screening method of a traditional Chinese medicine, and solves the problems of long time cycle, large consumption and high cost of screening of effective components of the artemisia capillaries soup in the prior art. The pharmacodynamic material basis screening method is as follows: 1, establishment of peak area of body components of the artemisia capillaries soup; 2, establishment of metabonomics biological markers of the artemisia capillaries soup on the basis of CCL4 liver injury model; and 3, establishment of plotting of correlation between marker metabolites and serum constituents (PCMS) research method. The invention provides the pharmacodynamic material basis screening method of the traditional Chinese medicine, the pharmacodynamic material basis screening method is fast, simple, accurate, low-cost, small in consumption, and better in versatility, can effectively determine the efficacy-related components, can reflect the efficacy index comprehensive effect characteristic of multi components, and can be used for pharmacodynamic material basis screening.
Owner:王喜军
Medicament for treating infectious diseases, preparation method and application thereof
ActiveCN102106914AReduce pathological damageImprove local microcirculationAntibacterial agentsOrganic active ingredientsCholic acidALI - Acute lung injury
The invention provides a medicament for treating infectious diseases, a preparation method and the application thereof. The medicament is prepared from scutellaria baicalensis, jasmine, erigeron breviscapus and cholic acid, and has the following effects: by aiming at the central link--inflammatory reaction of an infectious disease, removing harmful substances such as inflammatory mediators, free radicals and the like, and improving the local microcirculation state, thereby alleviating the pathological damages caused by inflammations; meanwhile, the medicament has the function of regulating the overall internal environment, and can effectively treat lower respiratory tract infection and acute lung injury induced by infection; and the effective substances and functional mechanism of the medicament are relatively clear.
Owner:KANGMEI PHARMA
Smilax extract, and preparation method and application thereof
InactiveCN102671059ASimple manufacturing methodEasy extractionAntibacterial agentsAntipyreticAdditive ingredientMedicine
The invention provides a preparation method of a smilax rhizome extract, the smilax rhizome extract prepared by the method and application of the smilax rhizome extract to preparation of medicines for preventing, relieving and treating inflammation. The content of total saponin ingredients in the smilax rhizome extract is high, the total saponin ingredients account for over 50% of the weight of the smilax rhizome extract, pharmacodynamics tests prove that the therapeutic effect of the total saponin ingredients is remarkable, the total saponin ingredients are a principal medical effect material basis for treating gynecological inflammation through smilax, and can serve as a raw material of new drugs for effective part of traditional Chinese medicines.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Preparation method for Chuzhou chrysanthemum extract powder
The invention provides a preparation method for Chuzhou chrysanthemum extract powder according to the characteristics of effective constituents contained in Chuzhou chrysanthemum, and the preparation method aims at a plurality of effective constituents of the Chuzhou chrysanthemum. A rotary evaporator is adopted to perform double extraction on the Chuzhou chrysanthemum, extracts of volatile oil and acetic acid are obtained, and then decoction dregs are added into ethanol water to perform extraction again with ultrasonic. Beta-cyclodextrin wrapping is performed on the volatile oil to obtain wrappage. Acid extract solution is concentrated, alcohol extract solution is concentrated after alcohol is recovered, two concentrated solutions are merged, and freeze-dried powder is obtained after the two concentrated solutions are frozen and dried. Eventually, the wrappage and the freeze-dried powder are porphyrized and mixed to obtain the Chuzhou chrysanthemum extract powder. According to the preparation method for the Chuzhou chrysanthemum extract powder, the extract method is selected aiming at the characteristics of the plurality of effective constituents of the Chuzhou chrysanthemum, and each effective substance is retained to the maximum extent; extraction time is short, the temperature for the whole process is smaller than or equal to 80 DEG C, damage to efficacy material is avoided, and the preparation method is high in efficiency, time-saving and labor-saving; and the extract powder can be used in place of the Chuzhou chrysanthemum, and can also serve as raw material for production of medicine and health care products.
Owner:时维静 +1
Preparation method and quality detection method of bupleurum oral solution
The invention discloses a preparation method of a bupleurum oral solution. The components of the preparation only contain one medical material, namely bupleurum. During preparation, a flocculant is used to remove impurity particles in the liquid medicine and the clear liquid which is obtained through centrifugal filtration is used to prepare the product. The method changes the previous train of thought that the water extraction and alcohol precipitation technology is adopted to clarify the liquid medicine, thus the production cost can be saved, resources and the environment can be protected, the basic effective substance of the original prescription is kept, the problem of the traditional technology that when the water decoction is filtered and concentrated to directly prepare, the finished product is stored for a long time to generate a lot of precipitate and have low clarity degree can be improved, the production efficiency can be obviously increased, the production cost can be reduced, the production process can be simplified, the prepared bupleurum oral solution preparation has high content of the effective component and the curative effect is more remarkable. In addition, theinvention also provides a detection method for measuring the component content of the bupleurum oral solution on the existing quality standard basis of the bupleurum oral solution, thus the inherent quality of the preparation can be better controlled and the quality supervision and detection can be performed effectively.
Owner:GUANGDONG ZHONGSHENG PHARMA
Biomedical line union device for administering pharmacological substances
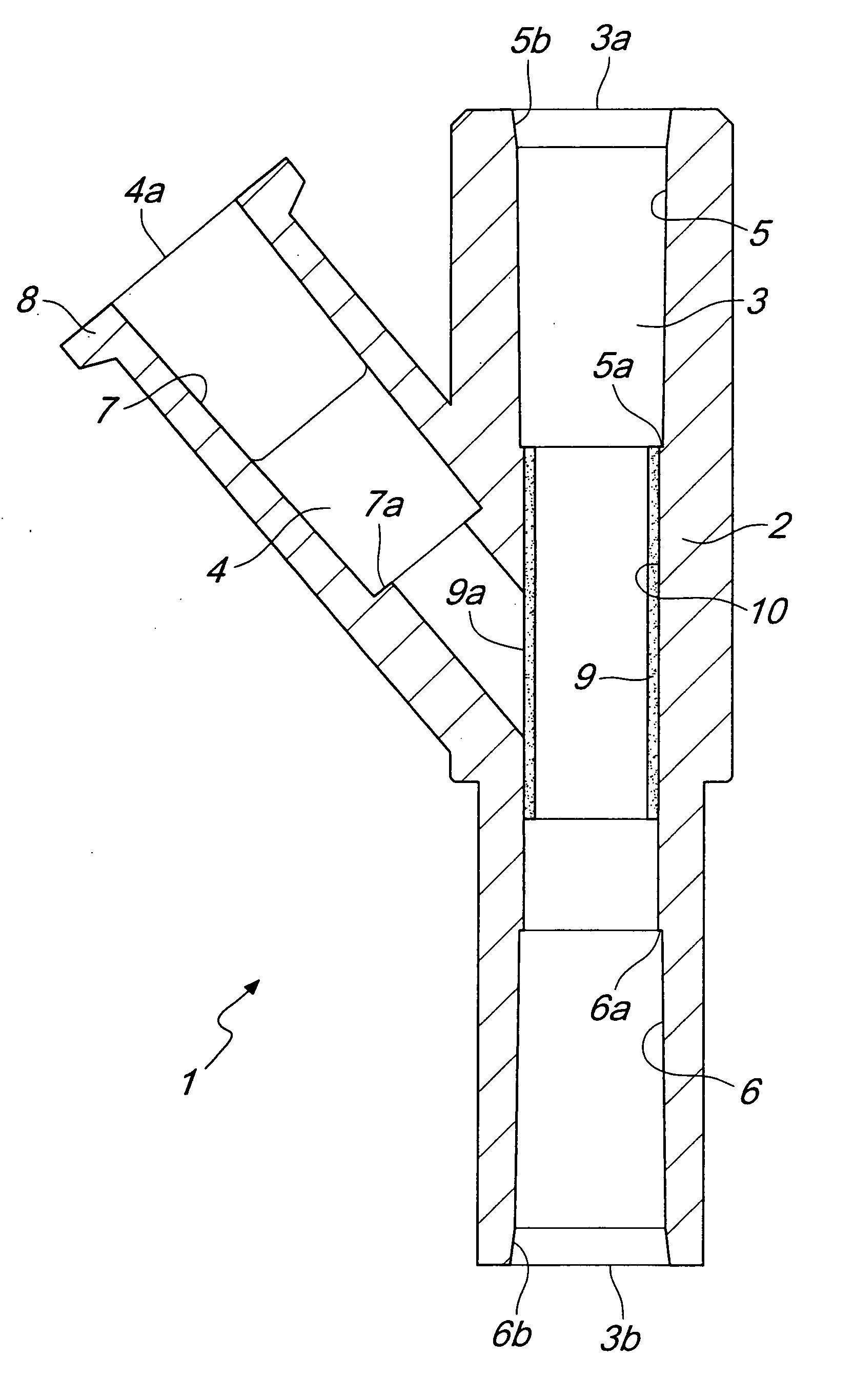
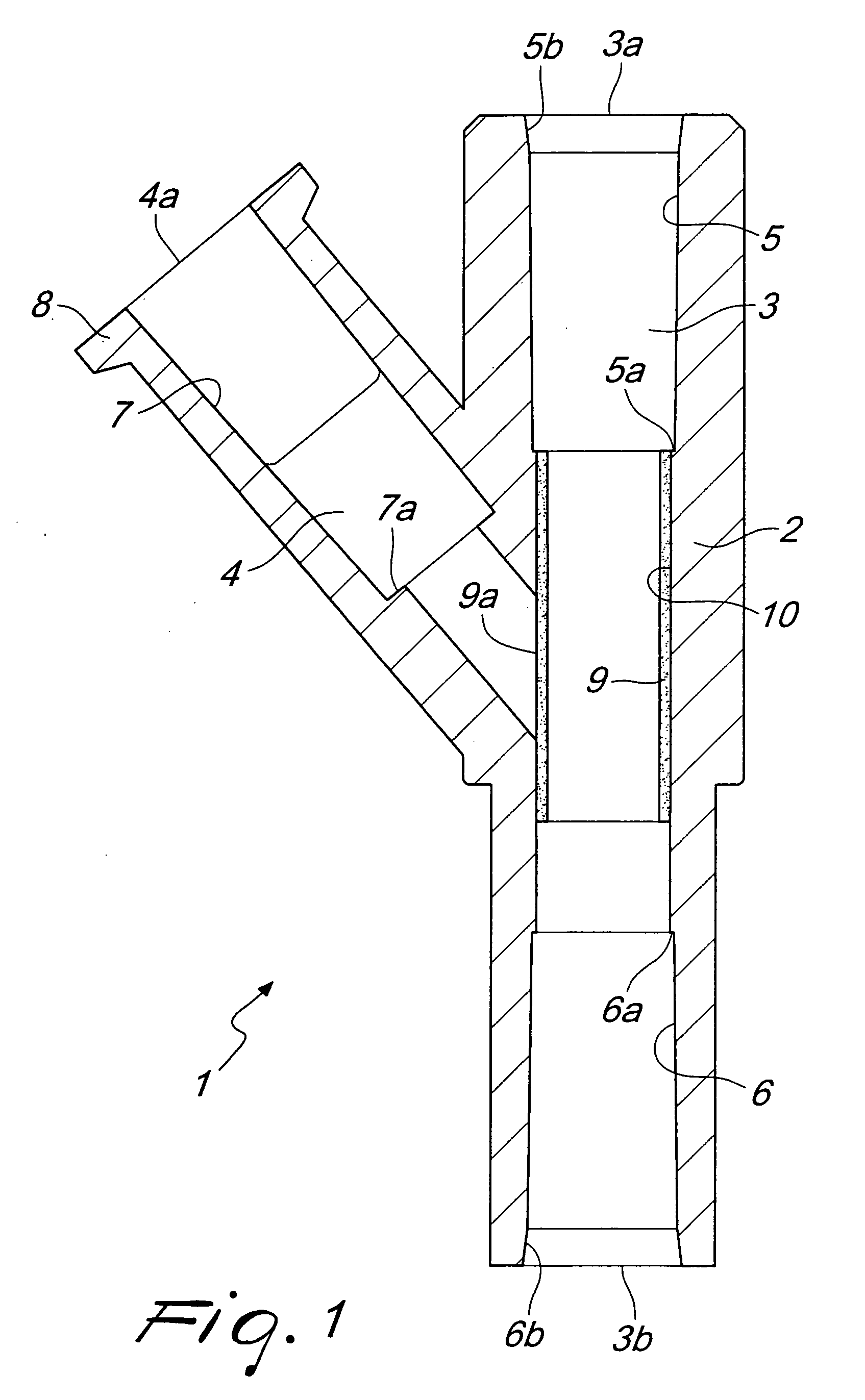
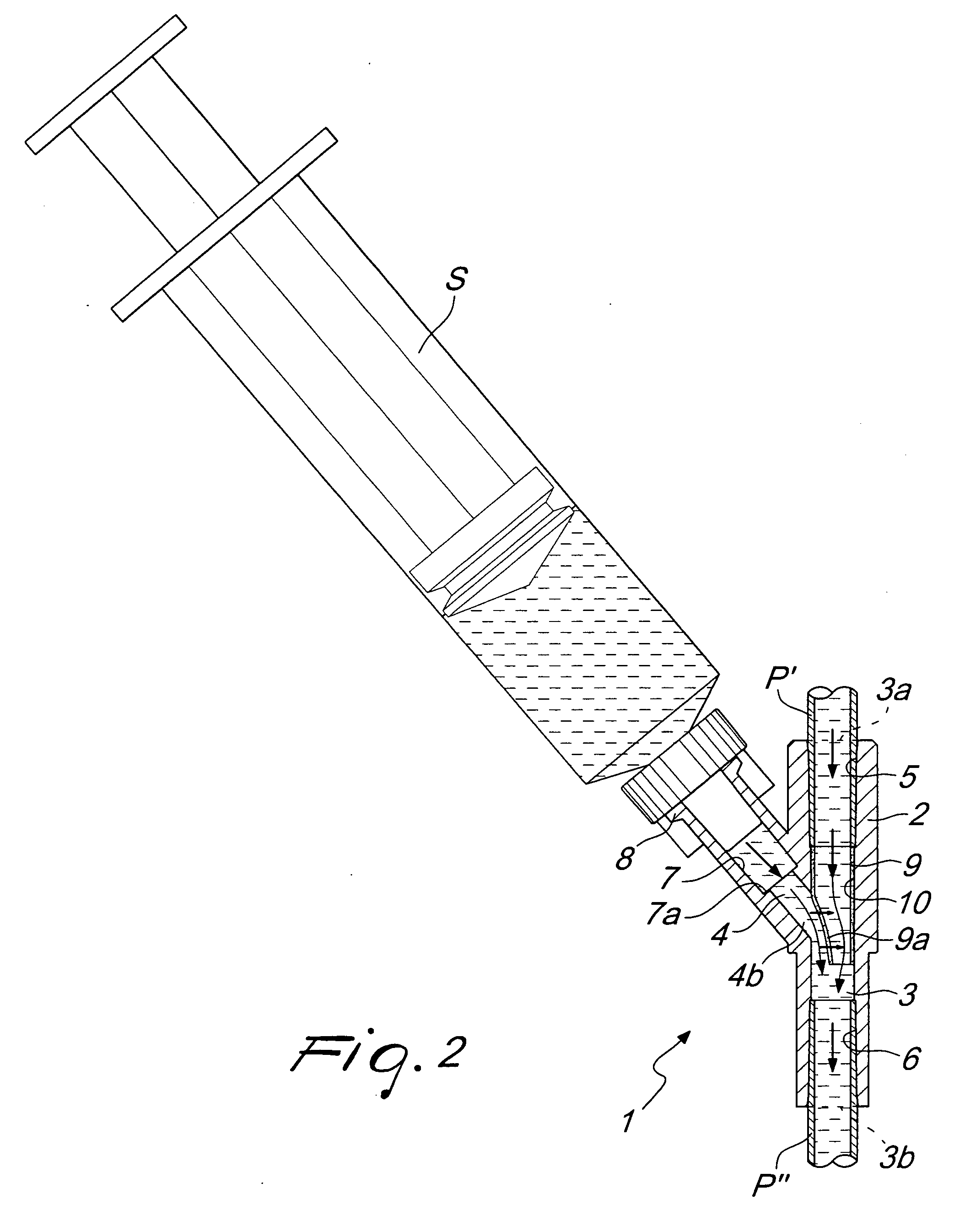
InactiveUS20090124983A1Improve personnel safetyEasy to provideIntravenous devicesCouplingsLine tubingPharmacological Substance
A biomedical line union device for administering pharmacological substances, comprising a union body with: at least one main duct thereinside, provided with at least one outlet associated with a conveyance line or with a closed container, and at least one branch duct, provided with at least one branching port associated with a line for administering a pharmacological substance and connected to the main duct by at least one connecting port. The device comprises at least one valve element, arranged proximate to the connecting port and movable between a closed configuration, in which it interrupts the connection between the branch duct and the main duct, and an open configuration, in which it provides the connection between the branch duct and the main duct due to an overpressure within the branch duct with respect to the pressure within the main duct, and vice versa.
Owner:LUCOMED
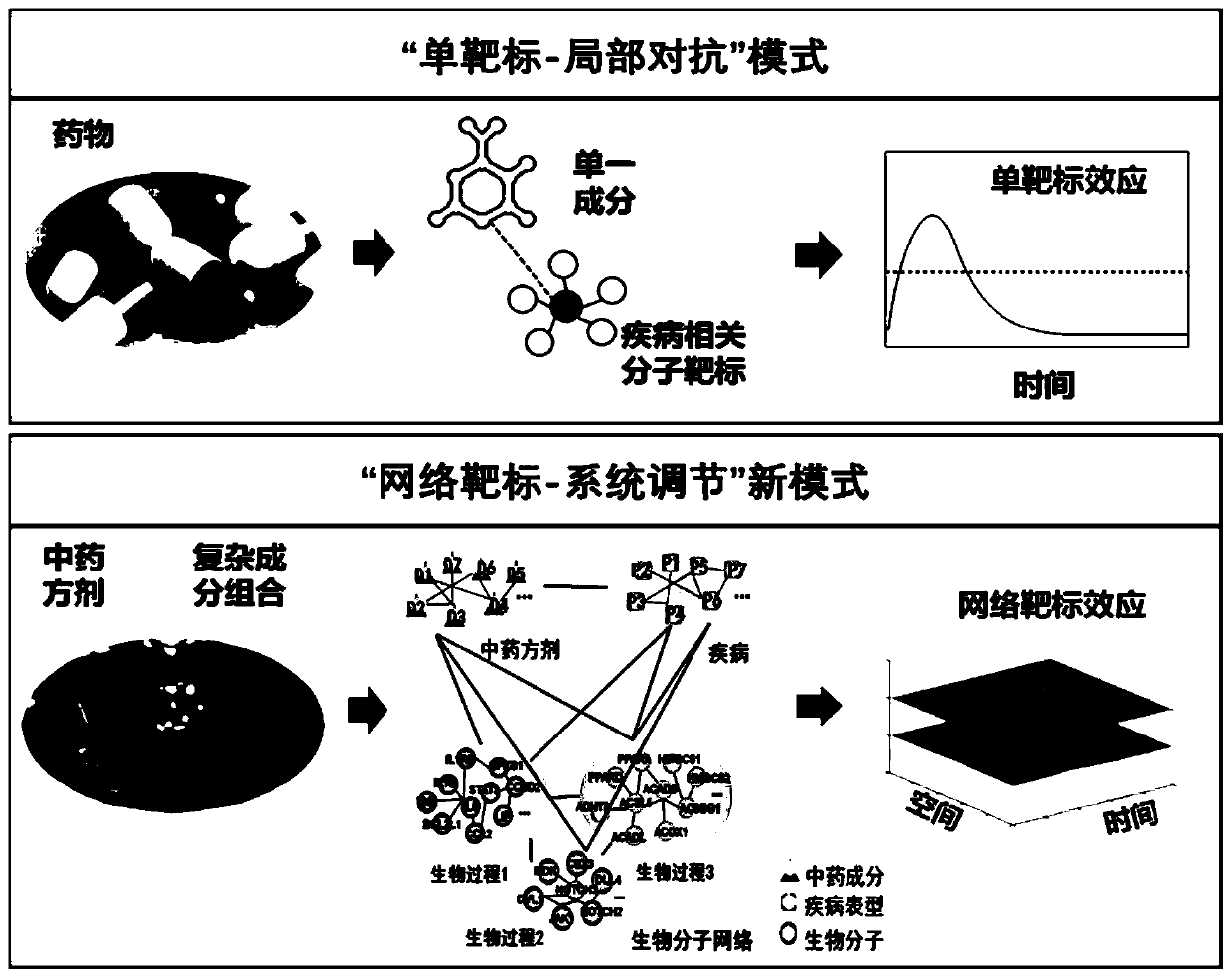
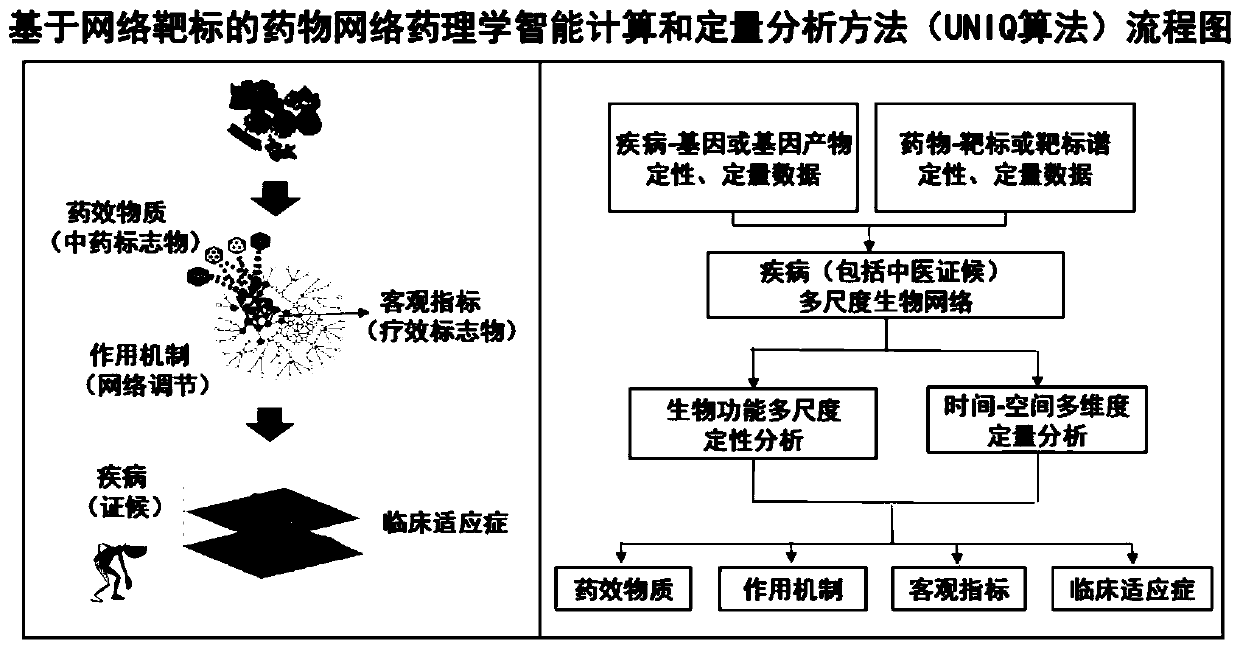
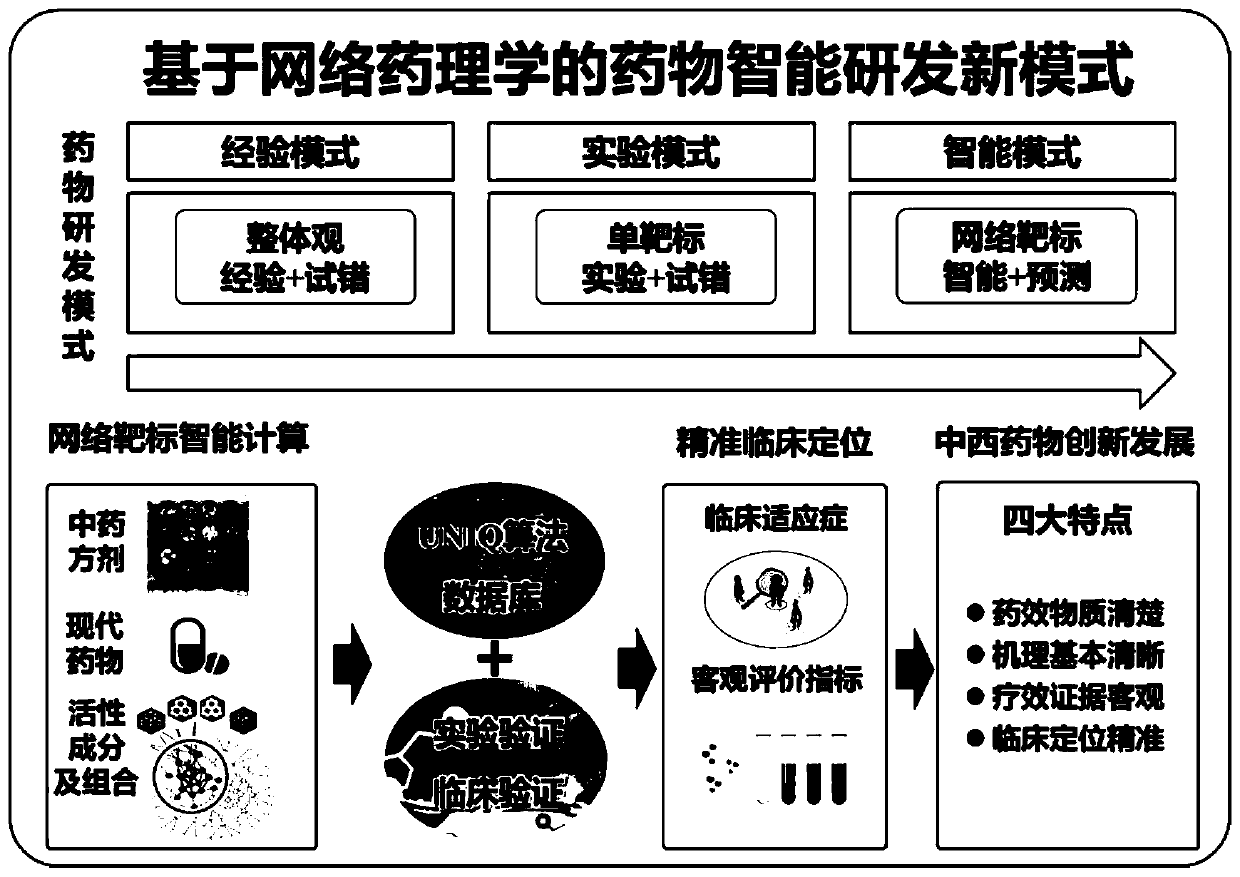
Drug network pharmacology intelligent and quantitative analysis method and system based on network target
The invention provides a drug network pharmacology intelligent and quantitative analysis method and system based on a network target original theory, wherein the method and system are used for measuring the overall effect of intervening a disease biological network by drugs (including various drug types such as Chinese and western drugs). The method can integrate qualitative and / or quantitative biological information related to diseases and drugs, takes the disease biological network as a target and realizes effect measurement of drug intervention on the disease biological network from the system and overall perspective, and reveals the overall action mechanism of the drugs. The method provides a qualitative and quantitative selectable measurement mode, measures the drug intervention network effect by adopting biological function multi-scale qualitative analysis and / or time-space multi-dimensional quantitative analysis, and provides a new key technique for breaking through the limitation of a traditional experience-based or single-target-based drug research mode, understanding a drug network regulation mechanism, and quickly and intelligently discovering pharmacodynamic substances, action mechanisms, curative effect objective indexes and clinical indications and the like.
Owner:TSINGHUA UNIV
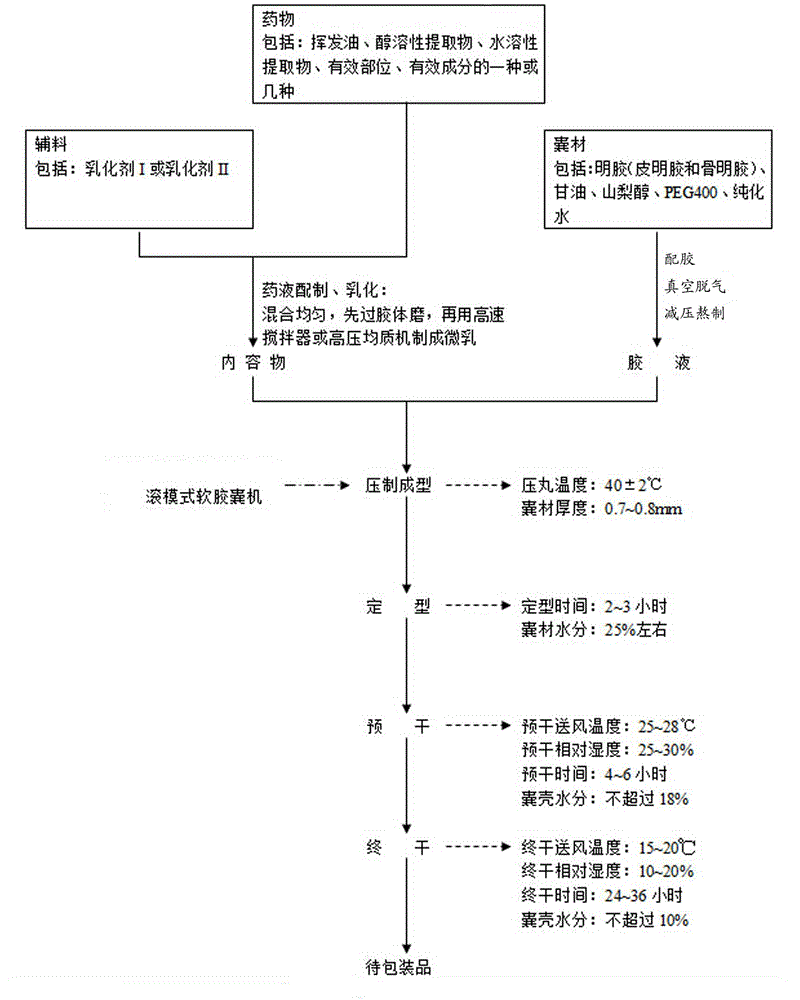
Traditional Chinese medicine soft capsule auxiliary material with universality and method for preparing traditional Chinese medicine soft capsules
InactiveCN104383551AIncrease the proportionReduce dosageCapsule deliveryOil/fats/waxes non-active ingredientsPharmaceutical drugEngineering
The invention relates to a traditional Chinese medicine soft capsule forming core technology with universality and a traditional Chinese medicine soft capsule forming core process with universality. The process consists of the following technologies: preparing the content and emulsifying, preparing a capsule wall material and melting, pressing and forming, and drying. The process comprises the following three aspects: 1, a mixed emulsifier used for the traditional Chinese medicine soft capsules and a preparation method thereof; 2, a capsule wall material formula used for the traditional Chinese medicine soft capsules and a preparation method thereof; and 3, technical parameters for pre-drying and final drying in the method for preparing the traditional Chinese medicine soft capsules. The application research proves that the application range of the traditional Chinese medicine soft capsules is widened; the specific gravity of the drug effect material is improved, and the amount of auxiliary materials is reduced; the drug stability is high, and phenomena of layering, leakage, deformation and transfer are avoided; the difference control level of the device is improved; and the disintegration time limit prolonging is effectively controlled.
Owner:山西黄河中医药研究所(有限公司)
Automatic analysis of cellular samples
The inventive method for automatically analysing a cellular sample consists (a) in colouring the cell nucleuses of a sample, (b) inobtaining at least one digital picture of the sample, and (c) in digitally analysing said image and is characterised in that the cell nucleus colouring stage (2) is embodied in the form of a DNA-non-specific colouring. In order to use the inventive method, said invention also relates to a method for selecting a culture medium, a method for measuring substance toxicity and to a method for measuring the cytopathological characteristics of a virus. A method for selecting pharmacological substances for treating excess weight and obesity diseases and a method for selecting substances for treating osteoporosis are also disclosed.
Owner:CELOGOS
Preparation method of total phenolic acid of erigeron breviscapus
InactiveCN103110680APromote absorptionEasy to makeCardiovascular disorderPlant ingredientsDiseaseDissolution
The invention discloses a medicinal plant extract and a preparation method and application thereof. The medicinal plant extract is powdery total phenolic acid or sodium salt thereof prepared by extraction, concentration, alkali dissolution and acid precipitation, degreasing and the like based on erigeron breviscapus as a raw material. The extract disclosed by the invention enriches various phenolic acid effective substances, the impurities such as pyromeconic acid, carbohydrates, oil and saponin are sufficiently removed, the active ingredients are comprehensive, and moisture absorption is avoided; and the extract can be independently used or combined with other medicinally-allowable substances and is used for preparing medicines and healthcare products for preventing and controlling ischemic cardiovascular and cerebrovascular diseases.
Owner:黄睿
Application of cinnamon polyphenol composition
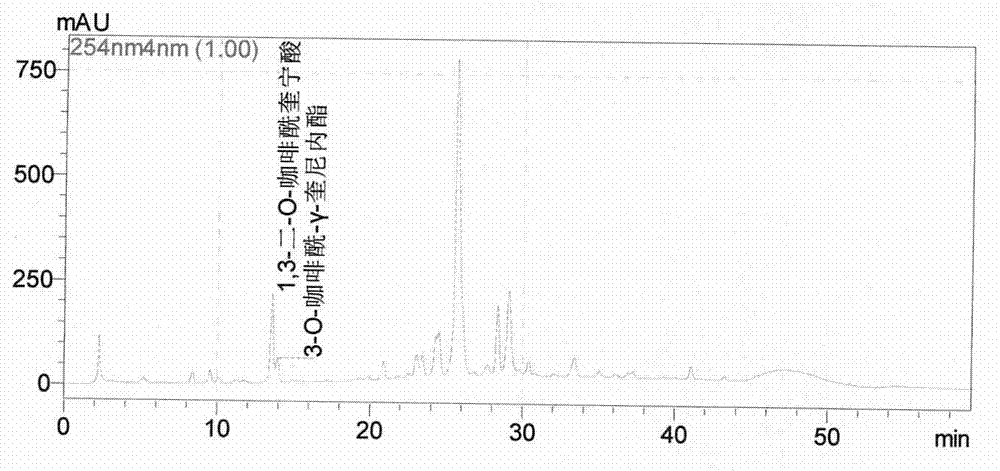
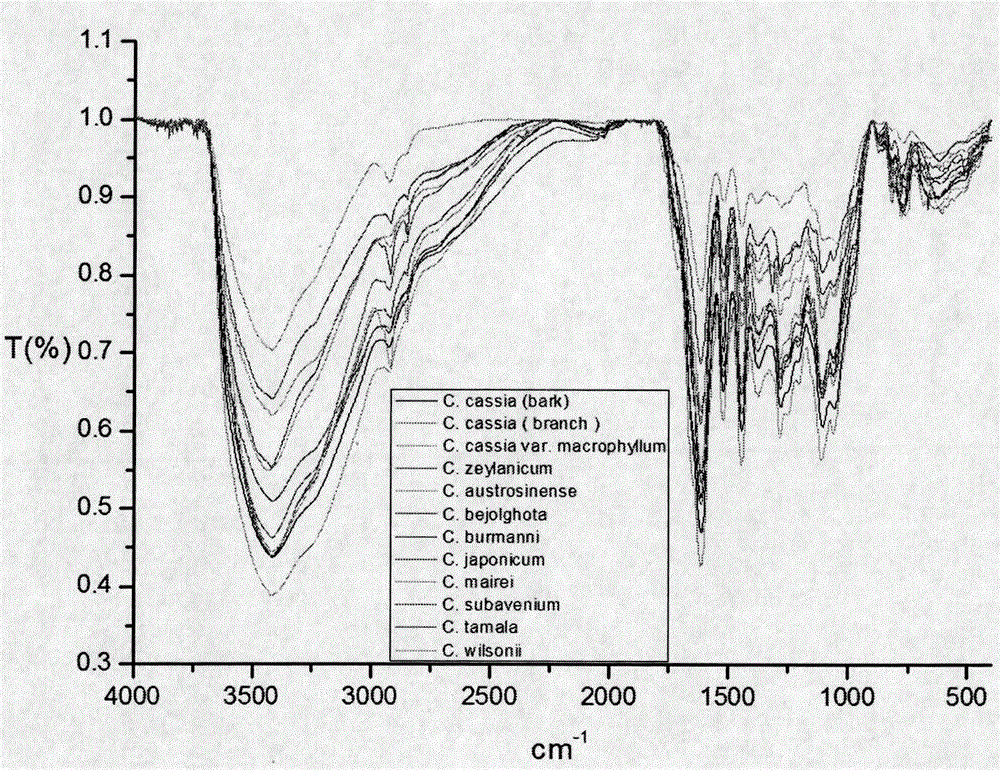
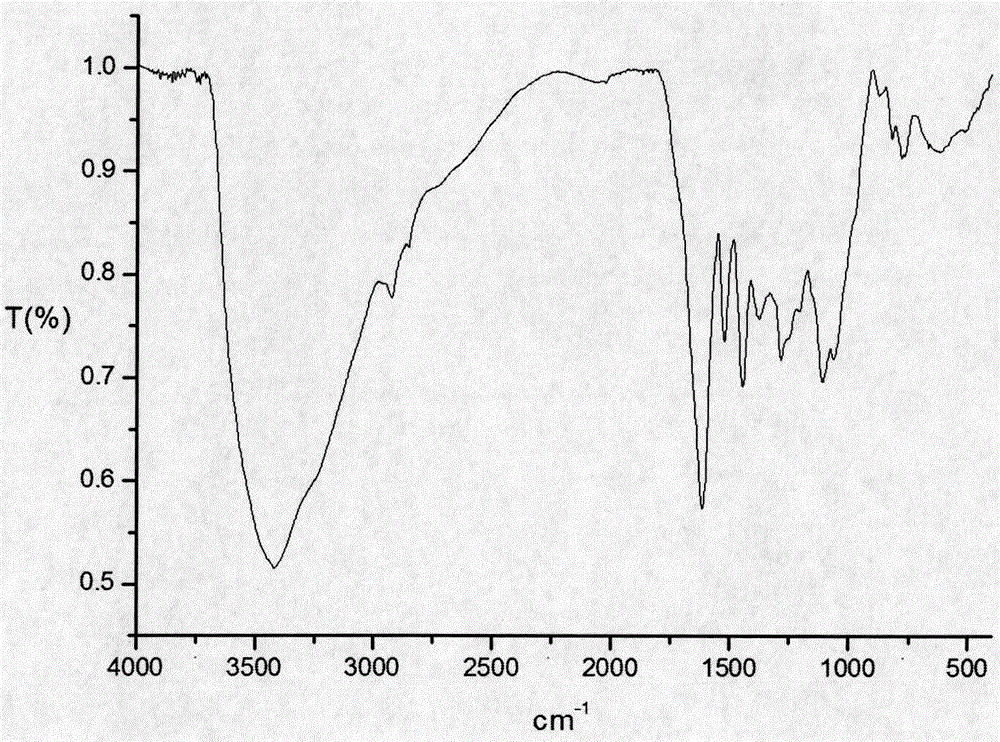
ActiveCN106606539AStrong antiviral activityQuality is easy to controlAntiviralsPlant ingredientsDiseaseTannin
The invention discloses application of a cinnamon polyphenol composition. The cinnamon polyphenol composition can be applied to preparation of drugs and / or medical instruments for prevention and / or treatment of virus infection diseases. The invention discloses antiviral activity of the cinnamon polyphenol composition. Based on an intermediate infrared spectrum fingerprint and total tannin and total sugar content determination technology, a quality control method of the cinnamon polyphenol composition is established. Study confirms that the cinnamon polyphenol composition meeting the quality control requirement of the invention has significant antiviral activity. The cinnamon polyphenol composition provided by the invention has the characteristics of basically clear pharmacodynamic substances, controllable quality and stronger efficacy, and adapts to the process technology and quality standardization requirements of modern manufacturing industry.
Owner:KPC PHARM INC
Data analysis method
The invention relates to a pharmacological substance based on biologically active substances obtained from the plant Tribulus terrestris L. to be used as an agent reducing blood sugar, improving blood circulation and especially blood circulation in veins and capillaries of the limbs of diabetic patients, reducing bad cholesterol level, increasing good cholesterol concentration, maintaining cardiovascular and liver functioning with an additional prophylactic or healing effect on the immune system and the immune resistance. The pharmacological substance represents a combination of bio-active trivalent chromium and a complex of steroid saponins obtained from the plant Tribulus Terrestris l. and consisting of furostanols, spirostanols, sapogenins, sterols, flavonoids and other biologically active substances typical of this plant.
Owner:ALEXIEV BLAGOY PETROV
Application of trollius chinensis bunge extract in preparation of drugs for treating virus diseases
ActiveCN103623076ANon-addictiveReduce dosageAntiviralsPlant ingredientsDiseasePharmaceutical medicine
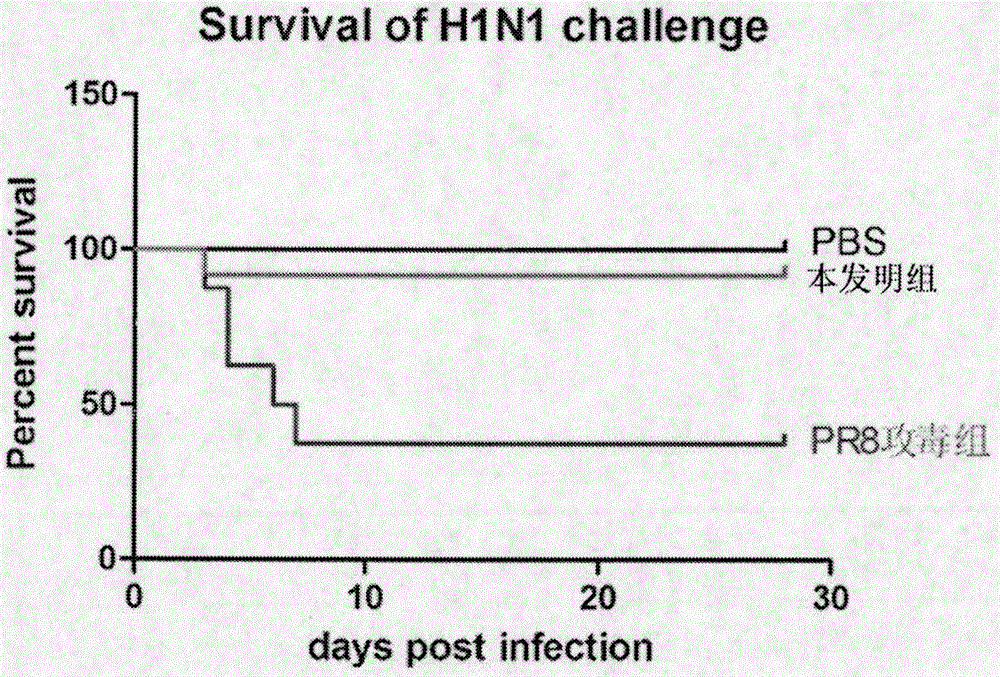
The invention discloses application of a trollius chinensis bunge extract in preparation of drugs for treating virus diseases. The purity of the trollius chinensis bunge extract is as follows: the total flavonoid content of the trollius chinensis bunge extract is higher than 50%. The trollius chinensis bunge extract is extracted from plants, traditional Chinese medicinal materials and Chinese herbal pieces all containing the trollius chinensis bunge. The trollius chinensis bunge extract can be applied to preparation of drugs for treating influenza virus H1N1, drugs for treating respiratory syncytial virus (RSV), and drugs for inhibiting the activity of and treating adenovirus type 3 (Adv3). The trollius chinensis bunge extract can be mixed with a barrier or an excipient acceptable on medicine to prepare the clinically acceptable medicinal preparation. According to the invention, the efficacy material for treating virus diseases from the traditional Chinese medicine, namely the trollius chinensis bunge extract, is found out, so that the scientific basis is provided for the clinical application of the extract, and the trollius chinensis bunge extract can be used as a natural antiviral agent, and has the characteristics of no addiction and smallness in dosage.
Owner:CHENGDE TIANYUAN PHARMACEUTICAL INDUSTRY CO LTD
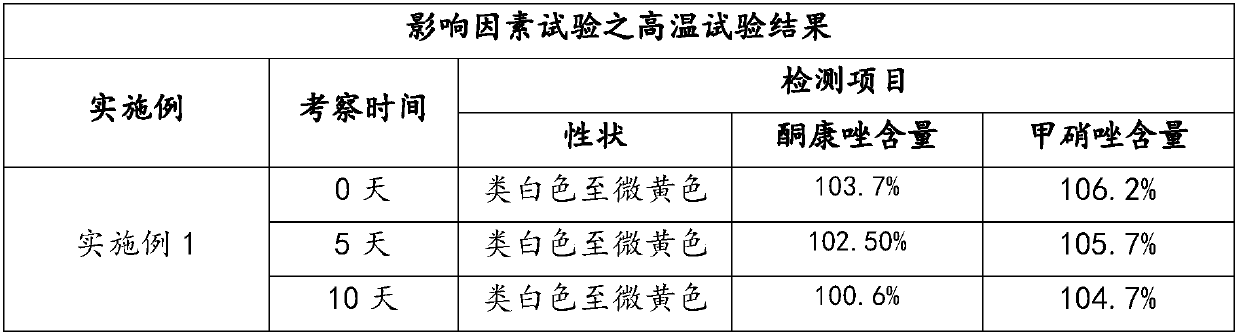
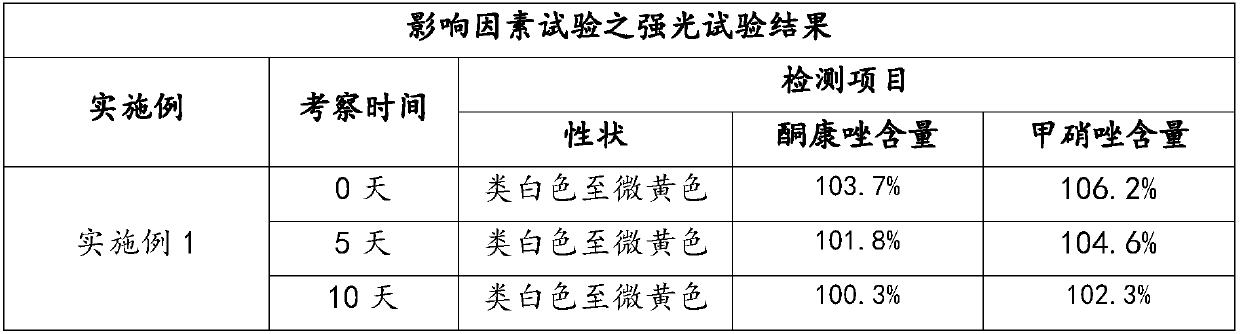
Preparation method and application of compound ketoconazole ointment
ActiveCN107625765AWell mixedImprove stabilityAntimycoticsHydroxy compound active ingredientsSodium sulfiteDrug
The invention provides a preparation method and application of compound ketoconazole ointment. The preparation method utilizes medicinal effective components such as ketoconazole, metronidazole and menthol and aids such as anhydrous sodium sulfite, a surfactant and hydrochloric acid as raw materials. Through adjustment and optimization of the preparation method, the compound ketoconazole ointmenthas stable performances and good bactericidal performances, effectively treats pet skin diseases and can be further used in preparation of a drug containing ketoconazole or metronidazole.
Owner:FOSHAN NANHAI EASTERN ALONG PHARMA CO LTD
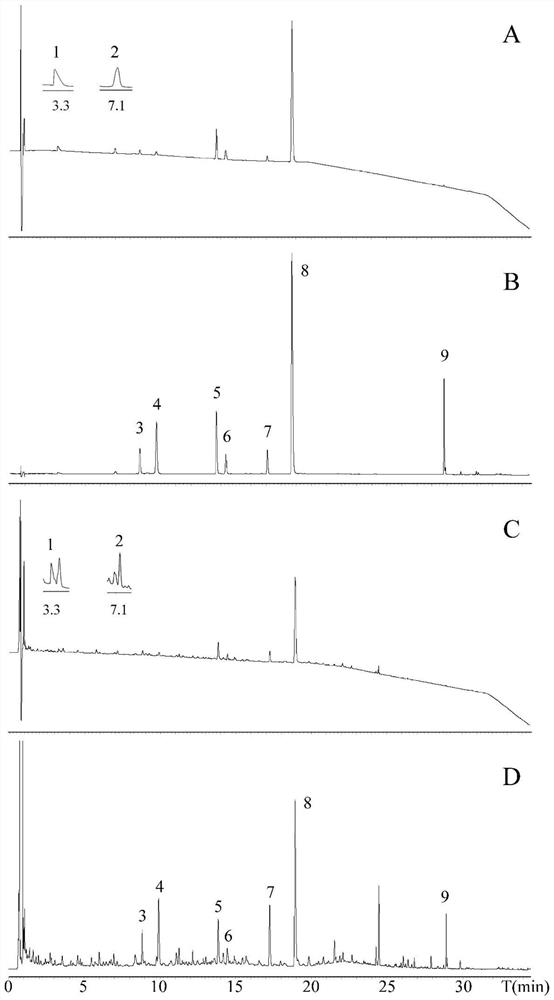
Method for identifying chemical components in Xuebijing injection
ActiveCN113791154AHigh sensitivityFast analysisComponent separationAgainst vector-borne diseasesFluid phaseChemical composition
The invention provides a method for identifying the chemical components in the Xuebijing injection. The ultra-high performance liquid chromatography-ion mobility-quadrupole time-of-flight mass spectrometry is combined, and the identification of multiple types of chemical components in the Xuebijing injection can be realized by reasonably selecting chromatographic conditions and mass spectrometry conditions; and the method has the advantages of simplicity, convenience, high sensitivity, high analysis speed, strong specificity and the like, and provides a basis for further researching the pharmacodynamic material basis of the Xuebijing injection.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
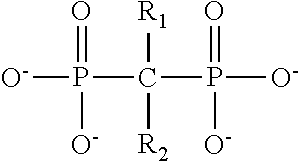
Digalactosyldiacylglycerol and preparation method and purpose thereof
ActiveCN106995474AReveal application valueOrganic active ingredientsSugar derivativesBranched chain fatty acidsFactor ii
The invention discloses digalactosyldiacylglycerol with a structure shown as a formula (I), a preparation method of the digalactosyldiacylglycerol and a purpose of the digalactosyldiacylglycerol for preparing a PPAR alpha agonist, or a PPAR gamma agonist and PPAR alpha / gamma double-agonists. The formula (I) is shown as the accompanying drawing, wherein R1 and R2 represent acyl fragments; the acyl fragments are straight-chain or branch-chain fatty acid containing 1 to 30 carbon atoms; in addition, 0 to 15 cis-form or trans-form double keys are contained in the acyl fragments. The agitation effect of digalactosyldiacylglycerol on PPAR alpha and PPAR gamma and the duplex agitation effect on PPAR alpha / gamma are discovered; the application value of the compound of the type to the prevention and treatment of PPARs relevant diseases is disclosed. In addition, active ingredients of alga beneficial to the cardiovascular health are discovered and separated; the medicine effect substances and functional factors are provided for developing alga medicine, health care food and food.
Owner:OCEAN UNIV OF CHINA
Analysis method for researching fingerprint spectrum of Mailuoning injection based on UPLC-DAD-MS
ActiveCN111239315AImprove quality control standardsOptimizing Chromatographic ConditionsComponent separationLignanDrug efficiency
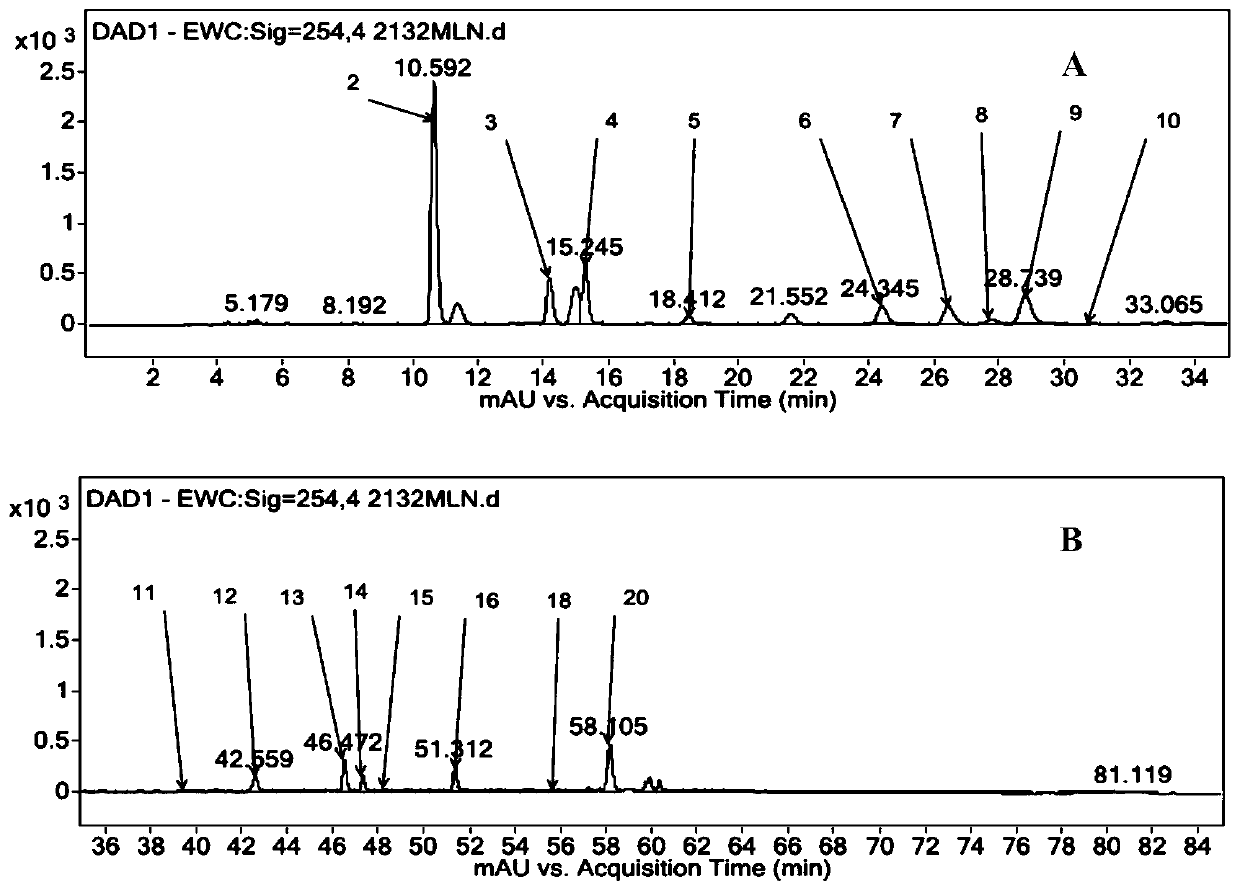
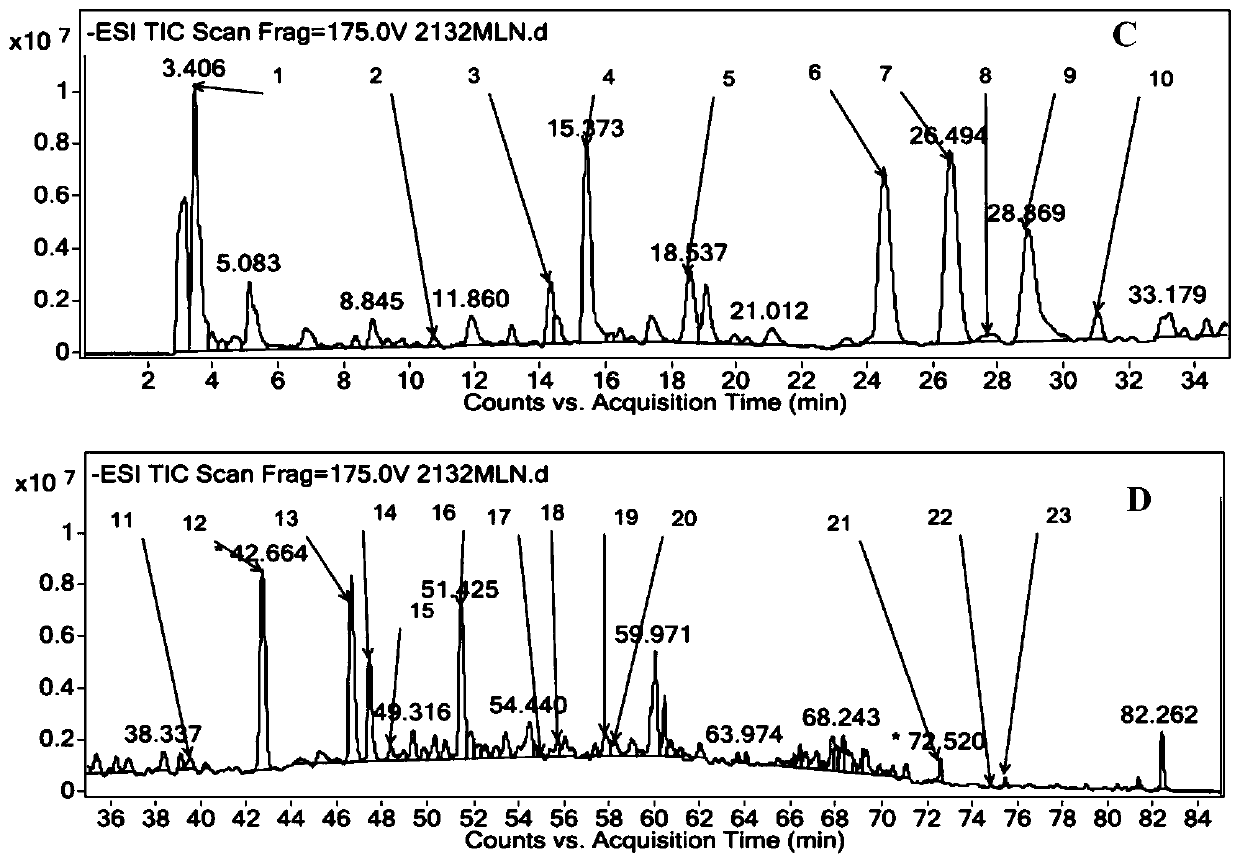
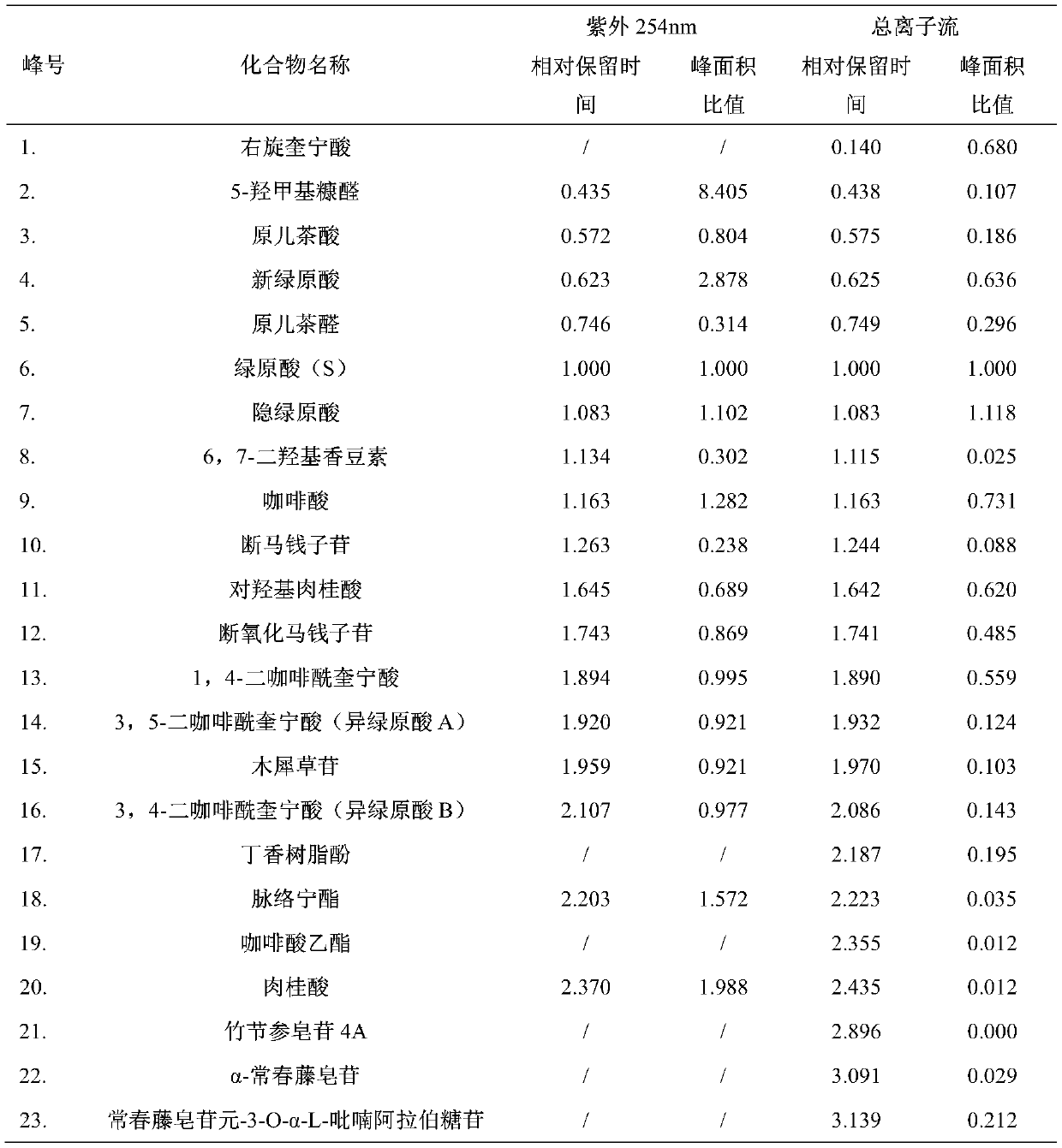
The invention discloses an analysis method for researching a fingerprint spectrum of a Mailuoning injection based on UPLC-DAD-MS. On the basis of research on early-stage chemical components, the UPLC-DAD-MS liquid chromatography-mass spectrometry fingerprint spectrum of the Mailuoning injection is established, and 23 main components including 13 organic acids, 1 coumarin, 2 iridoid, 1 flavone, 1 lignan, 3 saponins and 2 other components can be directly analyzed and identified. According to the method, optimized chromatographic conditions can be rapidly obtained; therefore, the optimal fingerprint spectrum is obtained, reproducibility is good, the relative retention time and the relative peak area variation are both less than 5%; the main chemical component composition of the Mailuoning injection is accurately illustrated, which is of great significance to illustration of the pharmacodynamic material basis of the Mailuoning injection, and lays a foundation for improvement of the qualitycontrol standard of the Mailuoning injection, establishment of a scientific and reasonable quality control method and optimization of a preparation process.
Owner:JINLING PHARMA
Method for evaluating quality of Herba Cirsii Setosi through quantitative analysis of multicomponents by single marker
InactiveCN109991327ASimplified Quantitative Assay MethodReduce testing costsComponent separationClinical efficacyActive component
The invention provides a method for evaluating quality of Herba Cirsii Setosi through quantitative analysis of multicomponents by a single marker. According to the method for evaluating the quality ofthe Herba Cirsii Setosi through the quantitative analysis of multicomponents by the single marker, firstly, a HPLC content determination method for four main medicinal components in the Herba CirsiiSetosi is established; rutin is used as an internal reference substance, relative correction factors of another three active components are calculated, and the system suitability and the method reproducibility of the relative correction factors are investigated; chromatographic peak positioning is carried out according to relative retention time, the relative correction factors are combined for calculating the content of all to-be-detected components, through the mutual verification of the quantitative analysis of multicomponents by the single marker and an external standard method, it is proved that no significant difference exists in determination results; the problems that reference substances are high in costs and are not easy to obtain are solved, the content of index components is calculated through the relative correction factors and the chromatographic peak positioning, and the simultaneous determination of multiple medicinal substances in the Herba Cirsii Setosi is realized; and the costs can be saved, the operation can be simplified, the efficiency is improved, the detection sensitivity is high, the stability is good, the determination results are exact and reliable, andthe method has great significance for ensuring the quality control and the clinical effects of the Herba Cirsii Setosi.
Owner:XIAN MEDICAL UNIV
Preparation process of heat-clearing and detoxifying veterinary traditional Chinese medicine
InactiveCN110882338AKeep drug activeAvoid deterioration and inactivationAntipyreticDrying solid materials without heatFreeze-dryingPharmaceutical Substances
The invention discloses a preparation process of a heat-clearing and detoxifying veterinary traditional Chinese medicine. The veterinary traditional Chinese medicine is prepared from the following components in parts by weight: 3-6 parts of folium isatidis, 5-7 parts of radix isatidis, 5-7 parts of gypsum, 5-6 parts of rheum officinale, 4-5 parts of compound of glauber-salt and liquorice, 4-6 parts of pericarpium citri reticulatae, 4-6 parts of astragalus membranaceus, 5-7 parts of scutellaria baicalensis, 3-5 parts of cinnamon, 3-6 parts of fried yam, 1-3 parts of codonopsis pilosula, 3-5 parts of Lysimachia christinae hance, and 3-5 parts of schizonepeta. By using a freeze dryer provided by the invention, the problems that an existing vacuum heating drying method enables active pharmaceutical substances with poorer heat resistance to lose activity, and volatile active pharmaceutical substances are easy to volatilize and lose in the heating drying process can be solved.
Owner:ANHUI HUAAO BIOTECH
Fingerprint spectrum construction method and application of Chinese herbal compound containing angelica sinensis
The invention discloses a detection method of a Chinese herbal compound containing angelica sinensis. The method comprises the following steps: taking a Chinese herbal compound test sample and reference substances for detection, wherein the Chinese herbal compound comprises angelica sinensis, cassia twig, licorice, white peony root, ginger and jujube; the reference substances are gallic acid, paeoniflorin, ferulic acid, liquiritin, ammonium glycyrrhizinate and 6-gingerol; and the chromatographic conditions of the detection are as follows: a C18 chromatographic column is adopted, methanol or acetonitrile is used as a mobile phase A, an acid aqueous solution is used as a mobile phase B, and a gradient elution procedure includes that 5%-12% A for 0-20 min is performed; 12%-85% A for 20-90 min is performed; a flow velocity is 0.8-1.2 mL / min; a column temperature is 25-40 DEG C; and a detection wavelength is 210-330 nm; and obtaining component information or component and content information of the Chinese herbal compound according to a detection result. According to the invention, the chemical components of the Chinese herbal compound containing the angelica sinensis are comprehensively and systematically analyzed by applying a high performance liquid chromatography technology, and a theoretical basis is provided for deep research on quality control and a pharmacodynamic material basis.
Owner:HUNAN YINENG BIOLOGICAL PHARMA
Method for establishing fingerprint spectrum of lung-ventilating and toxin-vanquishing prescription
ActiveCN112903845AAvoid one-sidednessScientific Evaluation QualityComponent separationFinger printPharmacological Substance
The invention discloses a method for establishing a fingerprint spectrum of a lung-ventilating and toxin-vanquishing prescription. The method comprises the following steps of: calculating RSD values of relative retention time and relative peak areaof each common peak by taking naringin as a standard substance; according to the invention, the fingerprint spectrum of the lung-ventilating and toxin-vanquishing prescription is established through the UHPLC method, and the contents of the nine effective components are determined, so that reference is provided for research on pharmacodynamic material basis and quality control of the lung-ventilating and toxin-vanquishing prescription.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
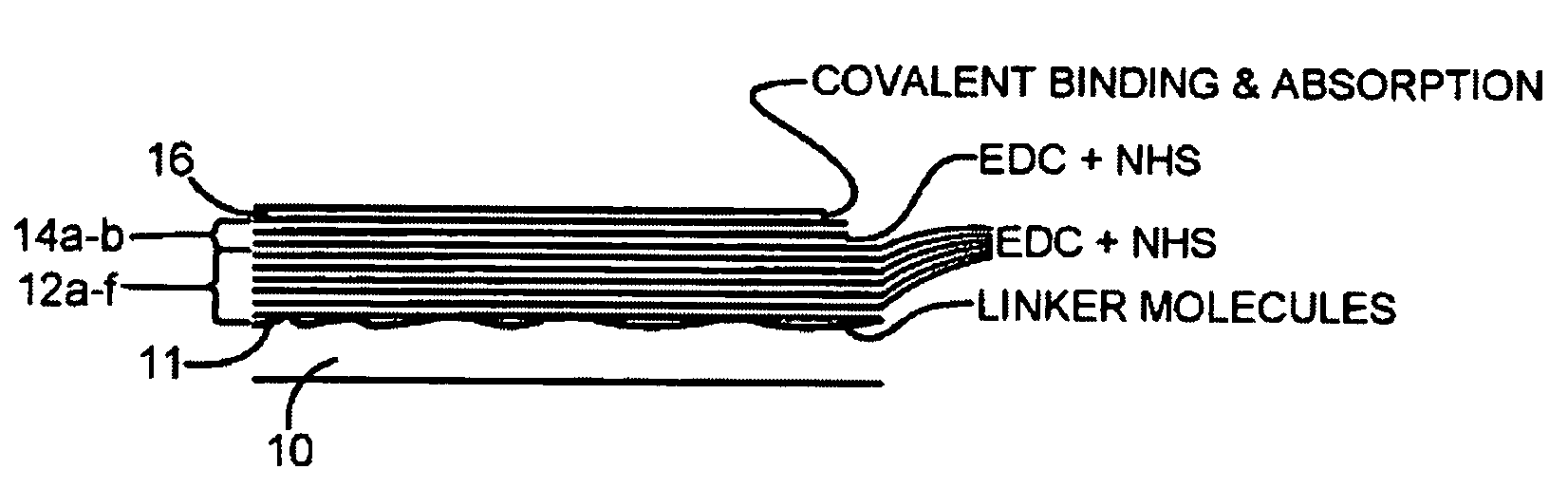
Biocompatible fibrinogen matrix on a solid support
The present invention provides a non-clottable matrix on a solid support comprising immobilized and crosslinked fibrinogen. The matrix may further comprise, in and / or on the matrix, one or several biologically active compound(s) and / or pharmacological substance(s). The matrix may be composed of one or several fibrinogen layer(s). The solid support according to the present invention may be selected from the group consisting of orthopaedic devices, implants, stitches, stents, pins, screws, plates, and sutures.
Owner:ADDBIO
Method for evaluating quality of gnaphalium affine by quantitative-analysis-of-multi-components-by-single-marker (QAMS) method
ActiveCN109991328ASimplified Quantitative Assay MethodReduce testing costsComponent separationChlorogenic acidClinical efficacy
The invention discloses a method for evaluating the quality of gnaphalium affine by a quantitative-analysis-of-multi-components-by-single-marker (QAMS) method. The method comprises the steps as follows: firstly, an HPLC content determination method for four main medicinal components in the gnaphalium affine is established; chlorogenic acid is used as an internal reference substance; relative correction factors of other three active components are calculated; the system applicability and the method reproducibility of the relative correction factors are inspected; chromatographic peak positioning is performed according to relative retention time; the content of each component to be determined is calculated in combination with the relative correction factors; and content determination resultsare proved to have no obvious difference by virtue of mutual verification of the QAMS method and an external standard method. The method disclosed by the invention solves the problems that the reference substance is high in cost and difficult to obtain; by virtue of the relative correction factors and the chromatographic peak positioning, the content of index components is calculated; synchronousdetermination of various medicinal substances in the gnaphalium affine is realized, so that the cost can be reduced, the operation is simplified, and the efficiency is improved; the method is high indetection sensitivity and high in stability; the determination results are accurate and reliable; and the method has a great significance on the quality control of the gnaphalium affine and the guarantee of the clinical effects.
Owner:XIAN MEDICAL UNIV
Method for carrying out quality control on platycodon grandiflorum (traditional Chinese herb) based on bioactivity assay
InactiveCN102419307AEasy to operateTest accurateColor/spectral properties measurementsBiotechnologySodium bicarbonate
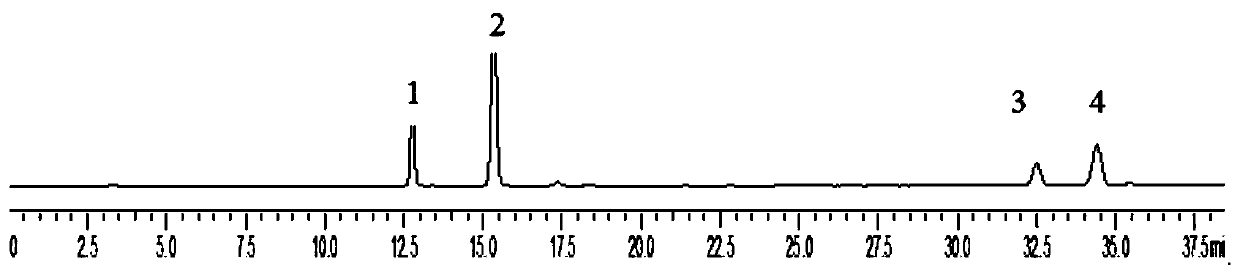
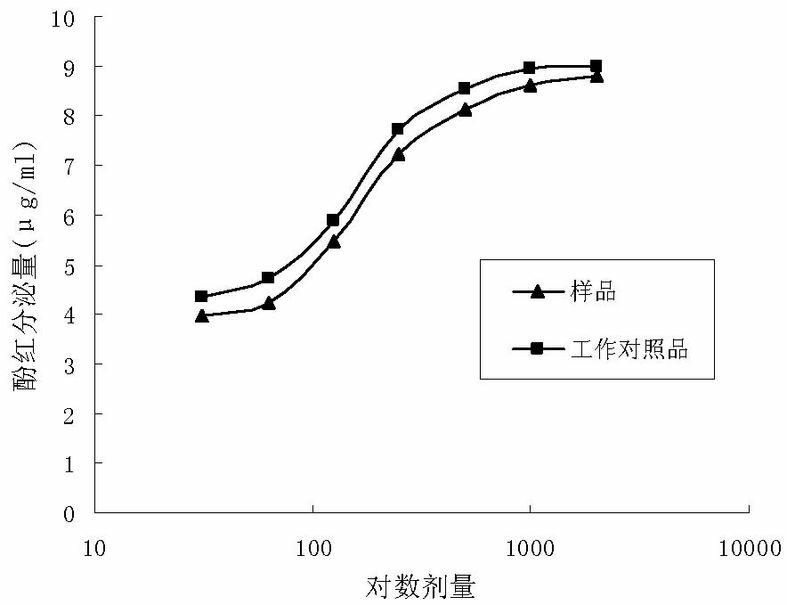
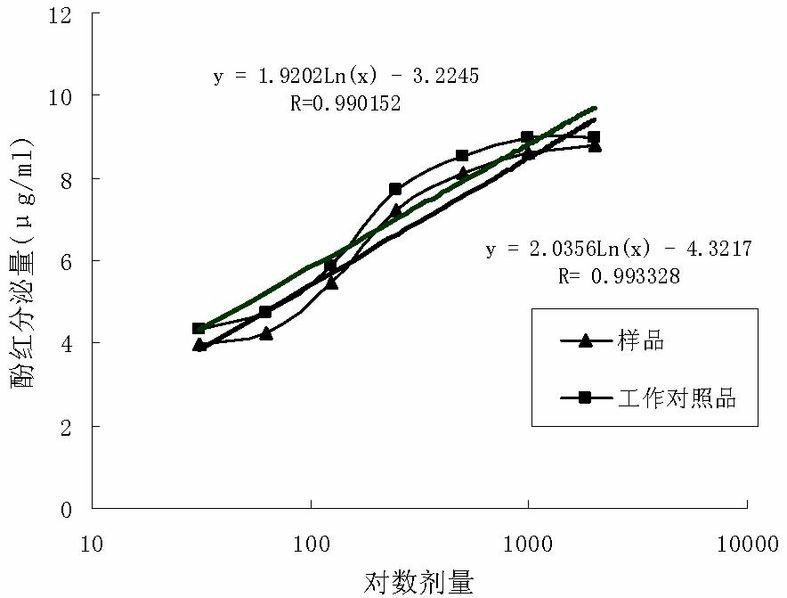
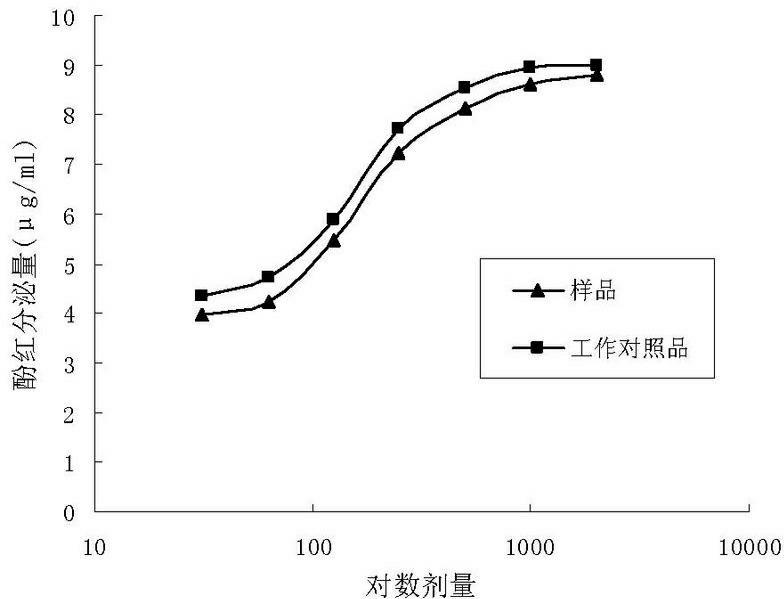
The invention relates to a method for carrying out quality control on platycodon grandiflorum (a traditional Chinese herb) based on bioactivity assay, which effectively realizes the quality control on platycodon grandiflorum and ensures the safe and effective clinical application of platycodon grandiflorum. The method comprises the following steps: crushing platycodon grandiflorum, and carrying out ultrasonic extraction on the crushed platycodon grandiflorum by using ethanol so as to obtain a dry matter; carrying out multiple proportion diluting on the obtained dry matter into seven groups by using normal saline according to an initial concentration at an agent distance of 0.5, thereby obtaining samples; preparing the platycodon grandiflorum into working reference substances according to the sample steps; dissolving phenol red by using a sodium bicarbonate solution, then making a standard curve through taking the dose of the phenol red as a x-coordinate and taking an OD (optical density) value as a y-coordinate; taking animals as experimental objects, grouping the animals, carrying out drug administration respectively, and putting the animals subjected to drug administration to death; then taking tracheas of the animals and putting the tracheas into normal saline, centrifuging and then taking supernate, determining the OD value at a wavelength of 546 nm, and calculating and comparing the secreted phenol-red content of the tracheas according to the phenol-red standard curve; and with the phenol-red content as an index, carrying out potency calculation. The method disclosed by the invention is easy to operate, stable, reliable, accurate in test and good in quality control effect, and can provide new ideas and references for the quality control and quality evaluation of other traditional Chinese herbs with undefined pharmaceutical active substances.
Owner:HENAN UNIV OF CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com