Digalactosyldiacylglycerol and preparation method and purpose thereof
A technology of lactosyl diacylglycerol and acyl, applied in the field of preparation of digalactosyl diacylglyceride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Extraction, preparation and structure identification of digalactosyl diacylglycerides
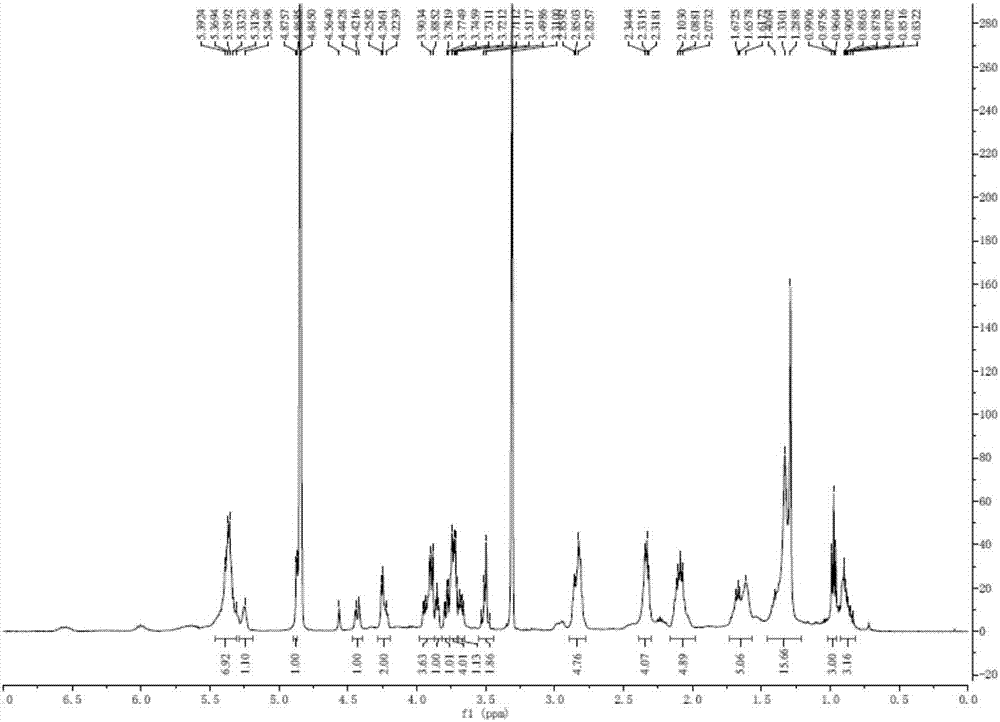
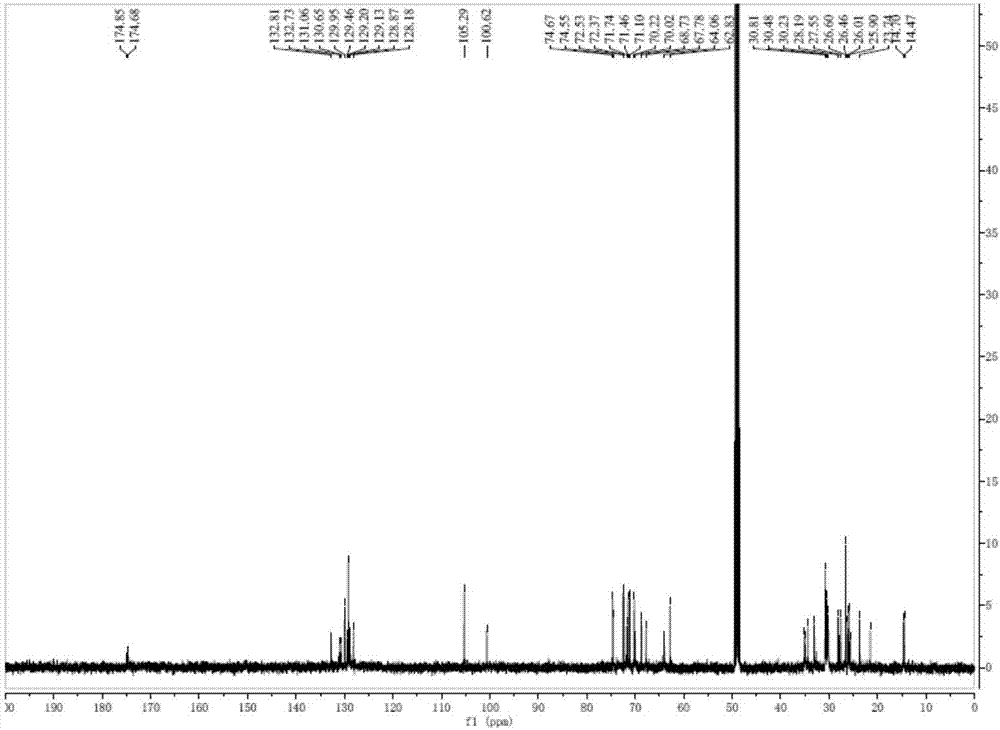
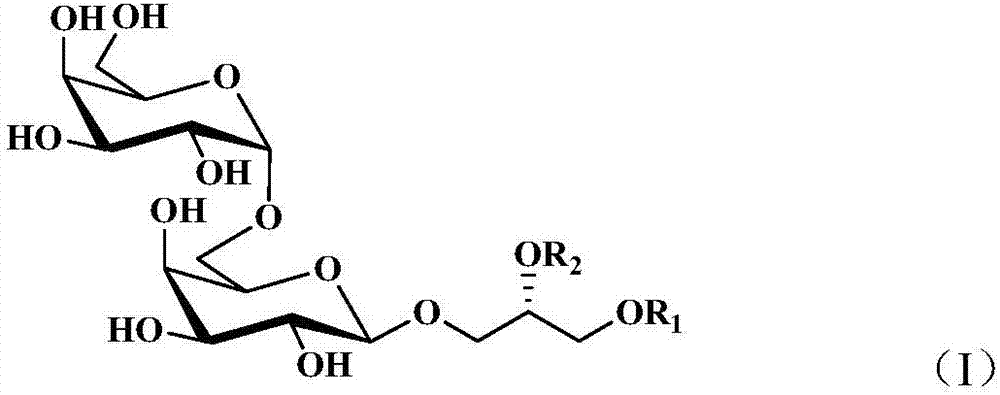
[0034] Take 2000 g of hijiki and extract with 10 times volume of 75% ethanol for 2 hours and repeat 3 times. The extracts were combined, filtered and concentrated until there was no alcohol taste, extracted with equal volume of ethyl acetate for 3 times, and the extracts were combined and concentrated to obtain 33.26 g of extract. The extract was dissolved in ethyl acetate, mixed with 41 g of 200-300 mesh silica gel H, and subjected to silica gel column chromatography, using dichloromethane-methanol as the solvent for gradient elution, where dichloromethane-methanol (v / v 85:15) ) The obtained components are eluted, and then subjected to Sephadex LH-20 gel column chromatography (dichloromethane-methanol 1:1) and silica gel column chromatography to obtain a mixture of digalactosyl diacylglycerides (DGDG) . Test it 1 H-NMR spectrum and 13 C-NMR spectrum (e.g. figure 1 with fi...
Embodiment 2
[0039] Example 2: The activation effect of DGDG on PPARα and PPARγ
[0040] The dual luciferase reporter gene analysis technique was used to detect the transcriptional activation of PPARα and PPARγ. Inoculate 293T cells in a 96-well plate with DMEM medium (10% FBS, without antibiotics). After 8-12 hours, the cells will grow to about 60%. Without changing the medium, transfect the plasmid directly according to lipo2000 instructions. The total amount of plasmid is 0.075g / well (0.05μg PPRE, 0.005μg internal control pRL-TK and 0.02μg PPARα / γ). The dosage of lipo2000 is 2.5 times the mass of the transfected plasmid (2.5*0.075L=0.1875μL / well). Plasmid and lipo2000 were mixed in 25μL / well optim medium. Twelve hours after transfection, a positive drug was added (the positive drug for PPARγ is rosiglitazone at a concentration of 1 μM, and the positive drug for PPARα is WY14643 at a concentration of 10 μM), and the DGDG obtained in Example 1. 24 hours after the addition of the drug, the...
Embodiment 3
[0041] Example 3: The activation effect of DD1~DD8 on PPARα and PPARγ
[0042] The dual luciferase reporter gene analysis technique was used to detect the transcriptional activation of PPARα and PPARγ, and the specific method was the same as in Example 2. The drugs added were DD1 to DD8 prepared in Example 1. The results are shown in Table 2.
[0043] Table 2 The activation effect of DD1~DD8 on PPARα / γ
[0044]
[0045] Note: Compared with the blank group, "*" P<0.05; "**" P<0.01; "***" P<0.001.
[0046] The results show that the above compounds can activate PPARα and / or PPARγ to varying degrees. In particular, DD8 (compound of formula (II)) exhibits significant PPARα / γ dual agonism.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com