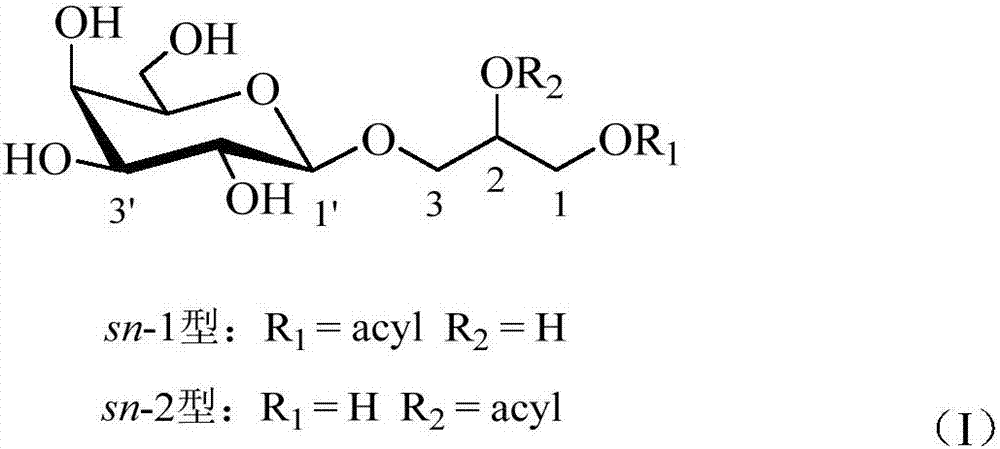
Preparation method and application of monogalactosyl-based monooleoyl glycerol
A technology based on monoacylglycerol and galactose, which is applied in the field of preparation of monogalactosyl monoacylglycerides, and can solve the problems of no MGMG and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Extraction preparation and structure identification of monogalactosyl monoacylglyceride
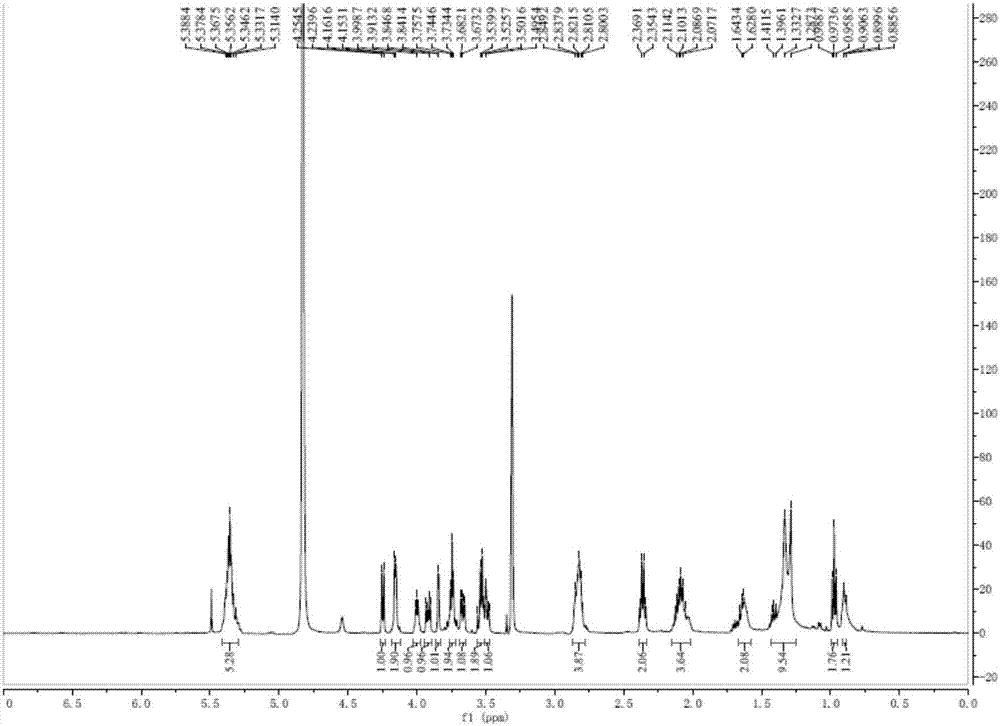
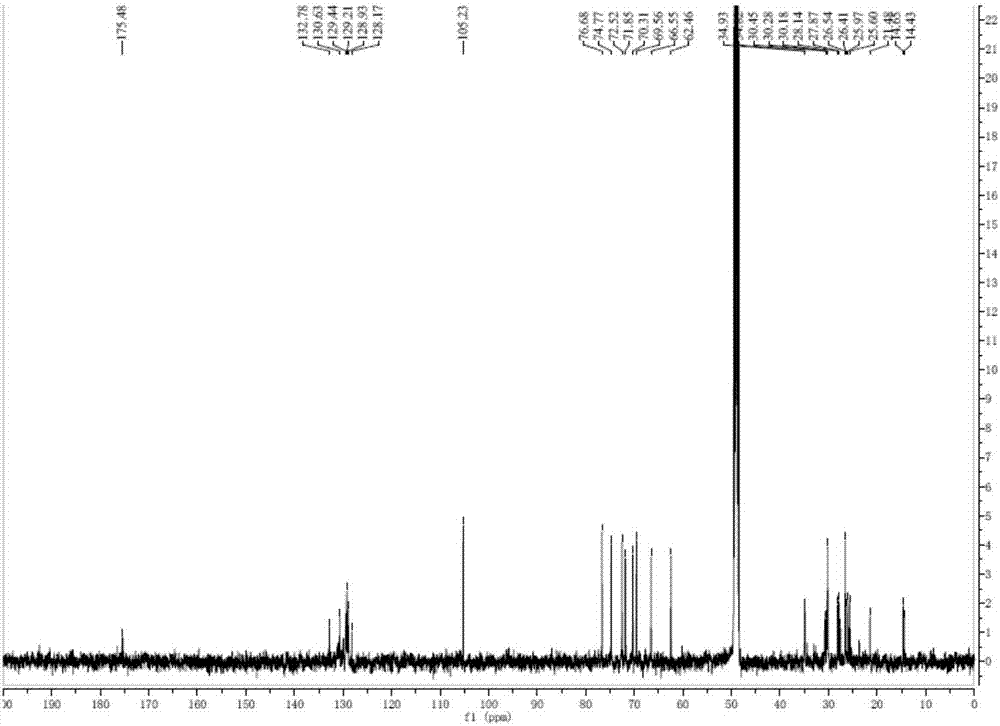
[0036]Take 2000g of Hijiki, extract with 10 times the volume of 75% ethanol under reflux for 2h, repeat 3 times. Combine the extracts, filter and concentrate until there is no alcohol smell, extract 3 times with an equal volume of ethyl acetate, combine the extracts, concentrate to obtain 33.26 g of extract. The extract was dissolved in ethyl acetate, mixed with 41g 200-300 mesh silica gel H, and subjected to silica gel column chromatography, using dichloromethane-methanol as solvent for gradient elution, wherein dichloromethane-methanol (v / v 90:10 ) to elute the resulting components, and then through Sephadex LH-20 gel column chromatography (dichloromethane-methanol 1:1) and silica gel column chromatography to obtain a mixture of monogalactosyl monoacylglycerides (MGMG) , measure its 1 H-NMR spectrum and 13 C-NMR spectrum (such as figure 1 with 2 shown).
[0037] ...
Embodiment 2
[0041] Example 2: Activation of PPARα and PPARγ by MGMG
[0042] Transcriptional activation of PPARα and PPARγ was detected using a dual-luciferase reporter gene assay. 293T cells were inoculated in 96-well plates with DMEM medium (10% FBS, without antibiotics). After 8-12 hours, the cells grew to about 60%. Without changing the medium, the plasmid was directly transfected according to the instructions of lipo2000. The total amount of plasmid was 0.075 g / well (0.05 μg PPRE, 0.005 μg internal control pRL-TK and 0.02 μg PPARα / γ). The amount of lipo2000 used was 2.5 times the mass of the transfected plasmid (2.5*0.075L=0.1875 μL / well). The plasmid and lipo2000 were mixed in 25 μL / well optim medium in advance. After 12 hours of transfection, the positive drug (the positive drug for PPARγ is rosiglitazone at a concentration of 1 μM, and the positive drug for PPARα is WY14643 at a concentration of 10 μM) and the MGMG obtained in Example 1 were added. 24 hours after adding the dru...
Embodiment 3
[0043] Example 3: Activation of MM1-MM7 on PPARα and PPARγ
[0044] The transcriptional activation of PPARα and PPARγ was detected by dual-luciferase reporter gene analysis technology, and the specific method was the same as that in Example 2. Added drugs are MM1-MM7 prepared in Example 1. The results are shown in Table 2.
[0045] Table 2 The activation effect of MM1~MM7 on PPARα / γ
[0046]
[0047] Note: Compared with the blank group, "*", P<0.05; "**", P<0.01; "***", P<0.001.
[0048] The results showed that the above compounds could activate PPARα and / or PPARγ to varying degrees. In particular, MM5 (compound of formula (II)) and MM6 (compound of formula (III)) exhibited significant PPARα / γ dual agonism.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com