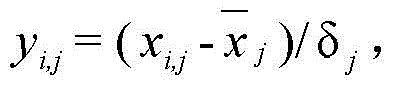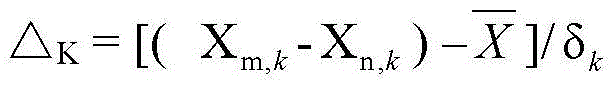Pharmacodynamic material basis screening method of artemisia capillaries soup
A kind of material basis, the technology of Artemisia capriculum, applied in the field of medicine, can solve the problems of long time period, high cost and large consumption of screening active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
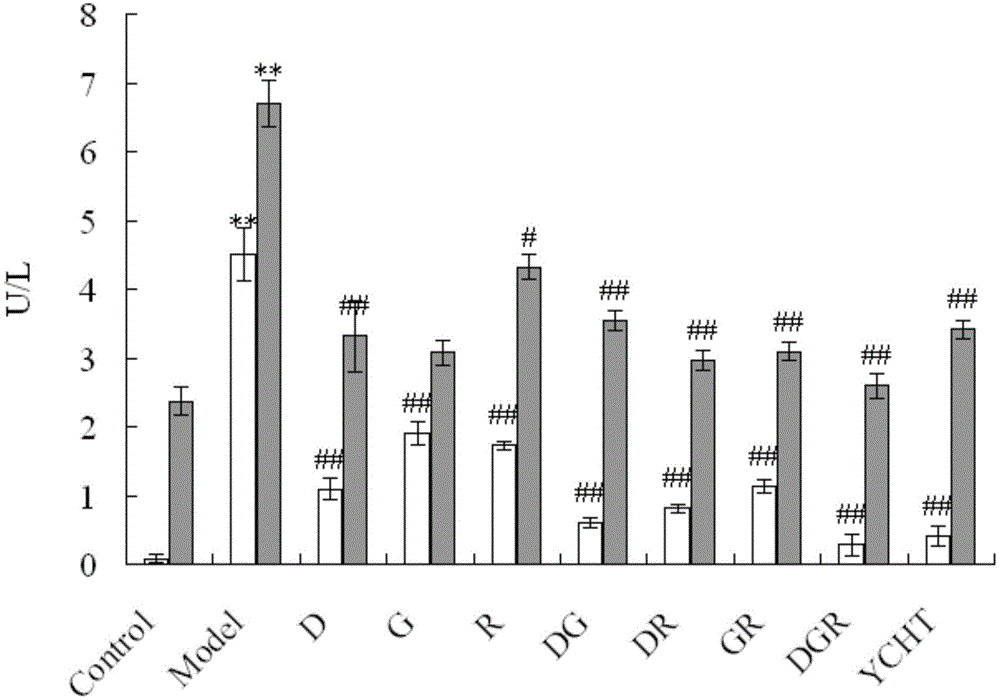
[0039] Embodiment 1: In this embodiment, the method for screening the effective substance basis of Yinchenhao Decoction is carried out according to the following steps:
[0040] 1. Establishment of peak areas of components in Yinchenhao Decoction:
[0041] (1) Preparation of the test sample: Weigh 18g of Artemisia chinensis, add 1400mL of water to boil, keep boiling and decoct to 700mL, add 9g gardenia and 6g rhubarb, keep boiling and decoct for 10min, filter the decoction with gauze, and concentrate the filtrate to The concentration of Artemisia annua is 1g / mL, and it is made into freeze-dried powder for later use;
[0042] (2) Preparation of samples for in vivo analysis: Add water to the freeze-dried powder prepared in step (1) to make a solution with a concentration of 0.65 g / mL, and give Wistar male rats intragastric administration at a dose of 1 mL / 100 g, and adopt the hepatic portal vein blood collection method Blood was taken 15 minutes after the administration, and th...
specific Embodiment approach 2
[0069] Embodiment 2: The difference between this embodiment and Embodiment 1 is that both the urine and blood samples are centrifuged at 4° C. and 10,000 rpm for 5 min as described in Step 2. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0070] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is: the high-performance liquid chromatography condition described in step two is: mobile phase is B phase 0.1% formic acid acetonitrile and A phase mass percent content is 0.1 % formic acid solution, the flow rate is 0.50mL / min. Others are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com