Powder injection of compound glycyrrhizic acid glycosides and preparation method thereof
A technology for glycyrrhizin and injection for injection, which is applied in the field of compound glycyrrhizin freeze-dried powder injection and its preparation, and can solve the problems of inability to fully meet, opalescence, strict requirements on storage conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
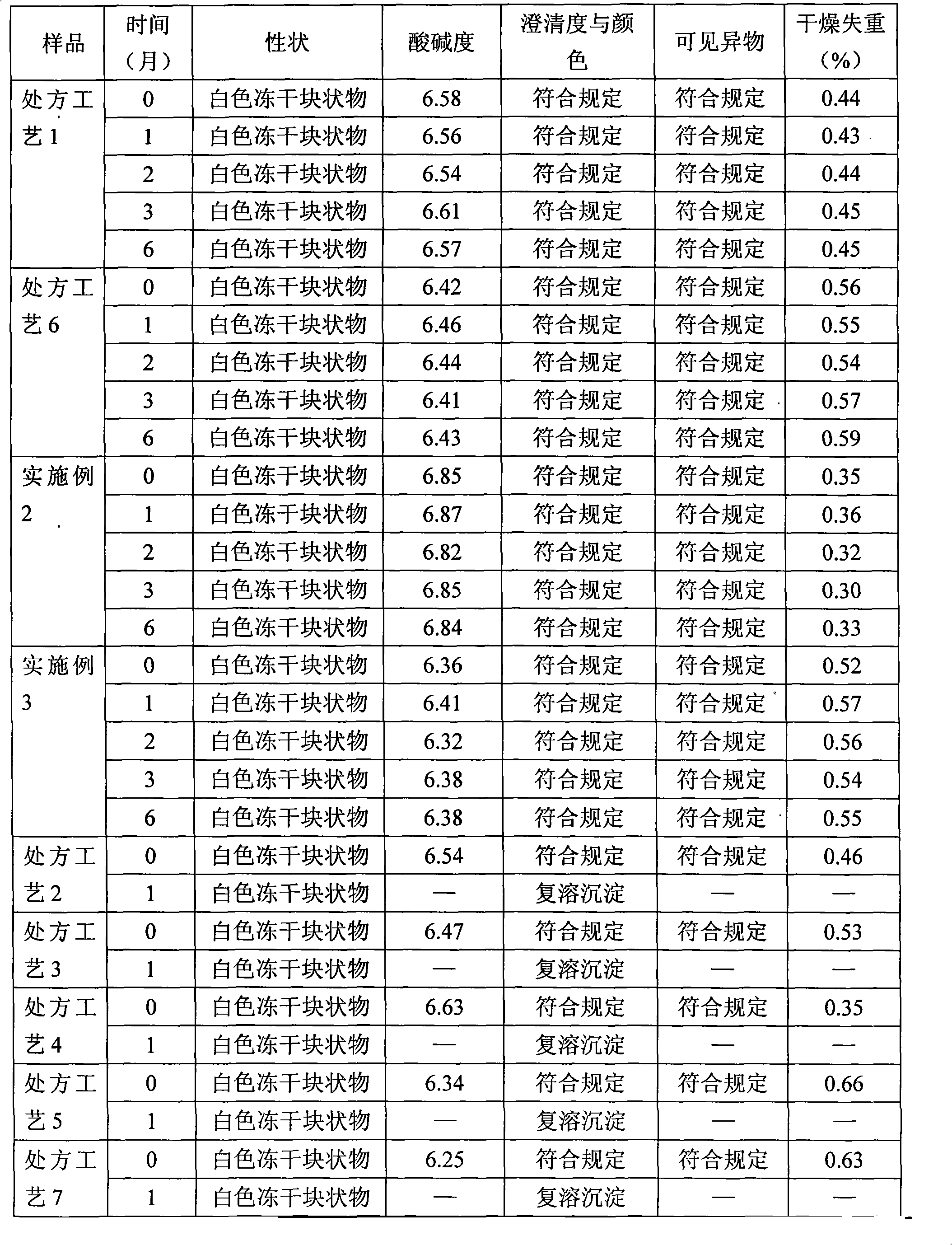
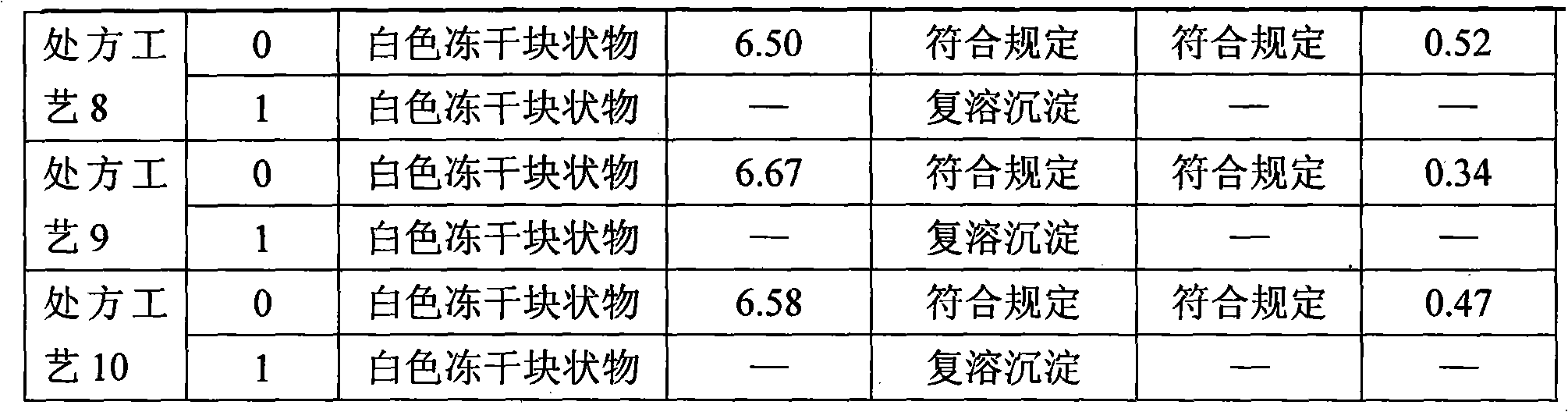
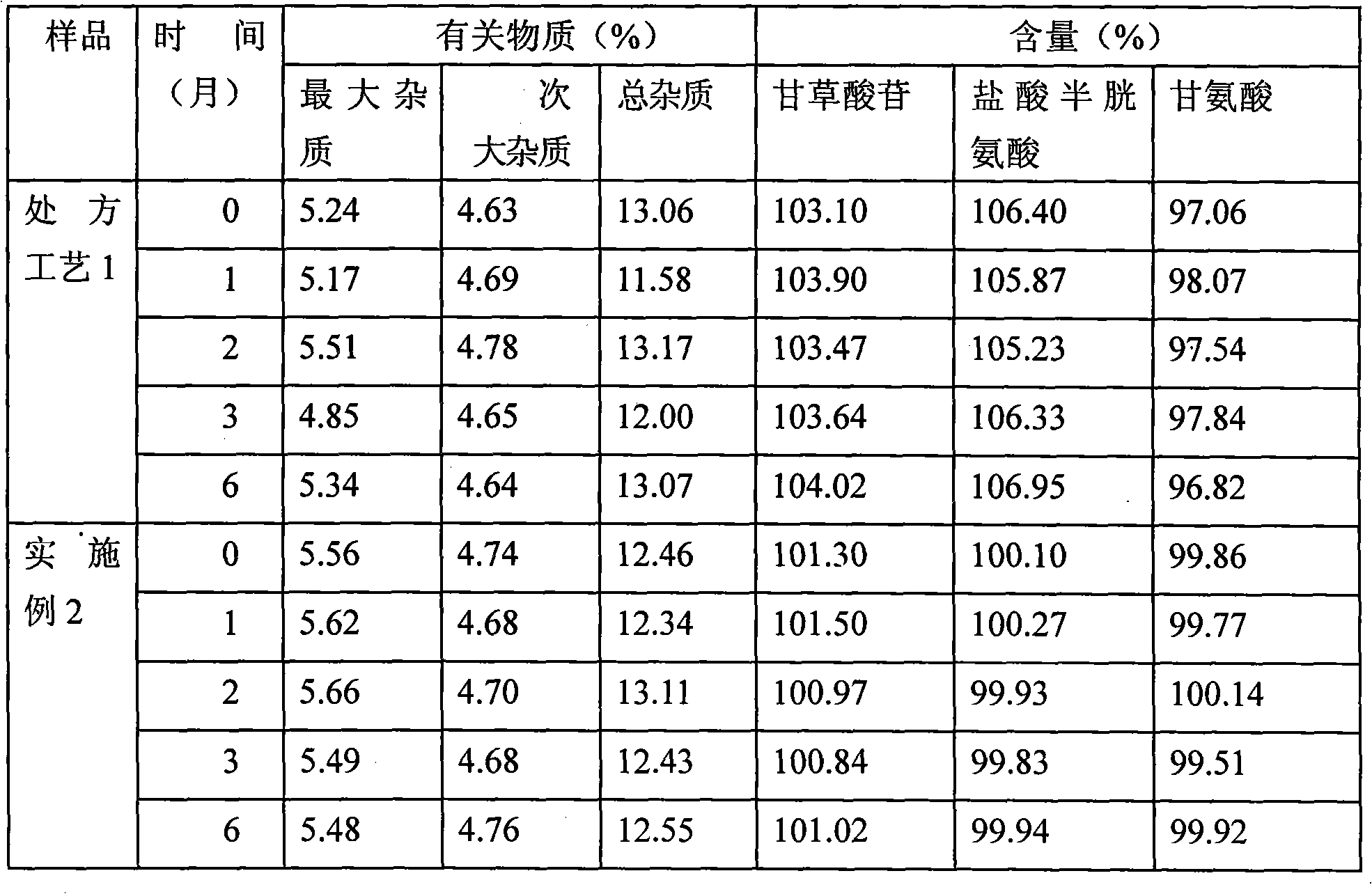
[0074] 1. Preparation of process samples of each recipe
[0075] 1.1 Prescription process 1
[0076] Prescription: monoammonium glycyrrhizinate S (calculated as glycyrrhizin) 400g, cysteine hydrochloride 200g, glycine 4000g, anhydrous sodium sulfite 160g, dextran 40500g.
[0077] Preparation method: Weigh dextran 40, add 10-15 times of water for injection, heat and stir to dissolve; add 0.2% (m / v) medicinal charcoal, stir for 20-30 minutes, decarbonize to obtain dextran 40 solution, take and prepare Measure 20% to 50% water for injection and dextran 40 solution, add it into the liquid mixing tank (barrel) and stir evenly; add glycine into the liquid mixing tank (barrel), stir to dissolve; dissolve anhydrous sodium sulfite with appropriate amount of water for injection, add the Stir in the liquid tank (barrel); add monoammonium glycyrrhizinate S into the liquid mixing tank (barrel), and stir until dissolved; dissolve the prescribed amount of cysteine hydrochloride with an ...
Embodiment 2
[0184] Prescription: monoammonium glycyrrhizinate S (calculated as glycyrrhizin) 400g, cysteine hydrochloride 200g, glycine 4000g, anhydrous sodium sulfite 160g, dextran 40430g, sodium chloride 75g.
[0185] Preparation method: Weigh dextran 40, add 10 to 15 times of water for injection, heat and stir to dissolve; add 0.2% (m / v) medicinal charcoal, stir for 20 to 30 minutes, decarbonize to obtain dextran 40 solution, take and prepare Measure 20% to 50% of water for injection and dextran 40 solution, add it into the liquid mixing tank (barrel) and stir evenly; add glycine into the liquid mixing tank (barrel), stir and dissolve; add anhydrous sodium sulfite and sodium chloride with appropriate amount of water for injection Dissolve, add to the liquid mixing tank (barrel), and stir; add monoammonium glycyrrhizinate S into the liquid mixing tank (barrel), and stir until dissolved; mix the prescribed amount of cysteine hydrochloride with an appropriate amount of water for inject...
Embodiment 3
[0187] Prescription: monoammonium glycyrrhizinate S 400g, cysteine hydrochloride 300g, glycine 4000g, anhydrous sodium sulfite 240g, dextran 40430g, sodium chloride 75g.
[0188] Preparation method: prepared with the same method as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com