Method of establishing fingerprint spectrum of infant diarrhea stopping drug preparation
A technology of pharmaceutical preparations and fingerprints, applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of lack of objectivity and accuracy in quality inspection results, inability to guarantee the reflection of Erxieting Granules and Erxieting Granules, and achieve Improve effectiveness and safety, ensure stability and uniformity, and reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
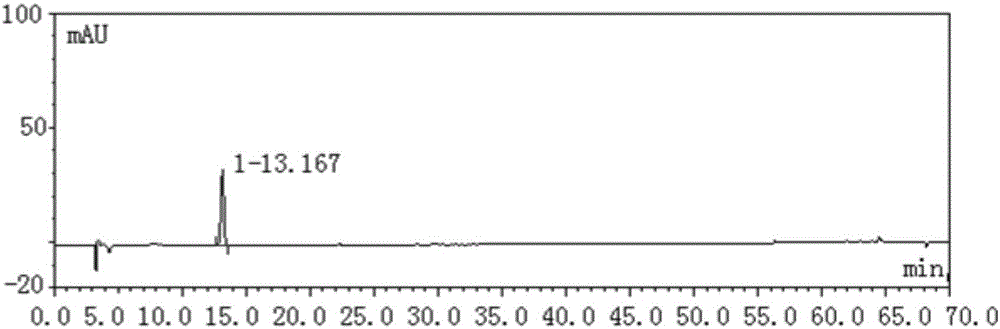
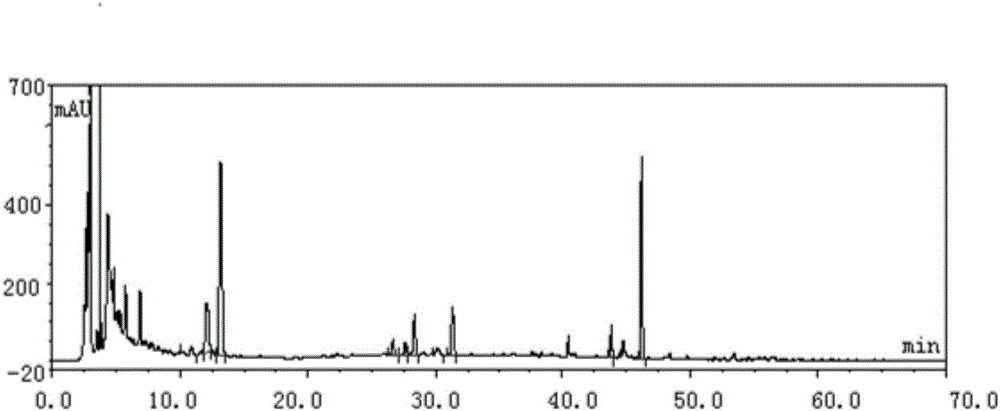
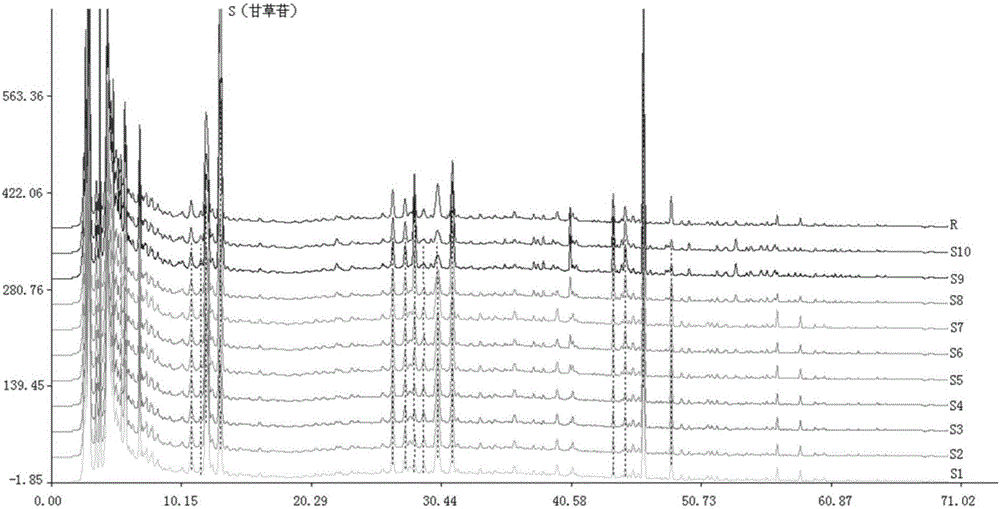
Image
Examples
Embodiment 1
[0046] In this embodiment, the method for establishing the fingerprint of Erxieting Granules comprises the following steps:
[0047] (1) Take 1.0 g of Erxieting granule to be tested, accurately weigh it, accurately add 25 mL of ethanol aqueous solution with a volume fraction of 70%, weigh it, extract it by ultrasonic for 30 minutes, let it cool, filter, and take the filtrate as the test product solution;
[0048] (2) Accurately weigh the reference substance of liquiritin, add methanol to make a solution containing 0.00010g per 1mL, shake well, and use it as the reference substance A solution;
[0049] Accurately weigh the ammonium glycyrrhizinate reference substance, add methanol to make a solution containing 0.00020g per 1mL, shake well, and use it as the reference substance B solution;
[0050] (3) Chromatographic conditions: use octadecylsilane bonded silica gel as filler, Techmate C18-ST with 250mm×4.6mm and 5μm as chromatographic column, acetonitrile as mobile phase A, a...
Embodiment 2
[0054] In this embodiment, the method for establishing the fingerprint of Erxieting Granules comprises the following steps:
[0055] (1) Take 1.0 g of the Erxieting granule to be tested, accurately weigh it, accurately add 25 mL of methanol aqueous solution or ethanol aqueous solution with a volume fraction of 80%, weigh it, perform ultrasonic extraction for 30 minutes, let it cool, filter, and take the subsequent filtrate, As the test solution;
[0056] (2) Accurately weigh the reference substance of liquiritin, add methanol to make a solution containing 0.00005g per 1mL, shake well, and use it as the reference substance A solution;
[0057] Accurately weigh the ammonium glycyrrhizinate reference substance, add methanol to make a solution containing 0.00010g per 1mL, shake well, and use it as the reference substance B solution;
[0058] (3) Chromatographic conditions: use octadecylsilane bonded silica gel as filler, Techmate C18-ST with 250mm×4.6mm and 5μm as chromatographic...
Embodiment 3
[0062] In this embodiment, the method for establishing the fingerprint of Erxieting Granules comprises the following steps:
[0063] (1) Take 2.0 g of the Erxieting granule to be tested, accurately weigh it, accurately add 50 mL of methanol aqueous solution or ethanol aqueous solution with a volume fraction of 40%, weigh it, perform ultrasonic extraction for 20 minutes, let it cool, filter, and take the subsequent filtrate, As the test solution;
[0064] (2) Accurately weigh the reference substance of liquiritin, add methanol to make a solution containing 0.00015g per 1mL, shake well, and use it as the reference substance A solution;
[0065] Accurately weigh the ammonium glycyrrhizinate reference substance, add methanol to make a solution containing 0.00030g per 1mL, shake well, and use it as the reference substance B solution;
[0066] (3) Chromatographic conditions: use octadecylsilane bonded silica gel as filler, Techmate C18-ST with 250mm×4.6mm and 5μm as chromatographic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com