Chiral tri-carbocyclic pyrimidine nucleoside analogue and preparation method thereof
A technology for carbocyclic pyrimidine nucleosides and analogues, applied in the field of chiral three-membered carbocyclic pyrimidine nucleoside analogues and their preparation, can solve the problems of complicated process and expensive raw materials, and achieve simple operation, easy availability of raw materials, and novel effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] Take a dry 100mL flask, dissolve pyrimidine (10.0mmol, 3.11g) and sodium tetrachloropalladate (1.0mmol, 294mg) in 185mL of vinyl acetate, reflux and stir at 80°C for 12h, then distill off the solvent under reduced pressure. The product was obtained by column chromatography with a yield of 88%. 1 HNMR (400MHz, CDCl 3 ): δ7.76(d, J=7.6Hz, 1H), 7.36(dd, J=8.8, 16.0Hz, 1H), 7.13(d, J=7.6Hz, 1H), 5.26(dd, J=2.0, 16.0Hz, 1H), 5.08(dd, J=2.0, 8.8Hz, 1H), 1.56(s, 18H); 13 CNMR (100MHz, CDCl 3 ): δ162.6, 153.3, 149.4, 142.4, 132.3, 104.5, 97.1, 85.2, 27.7.
Embodiment 2
[0030]
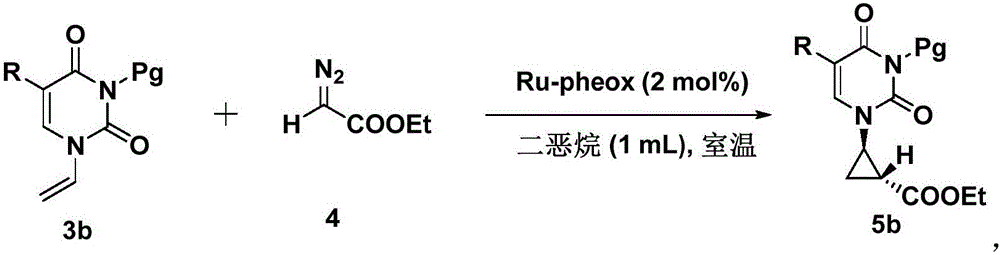
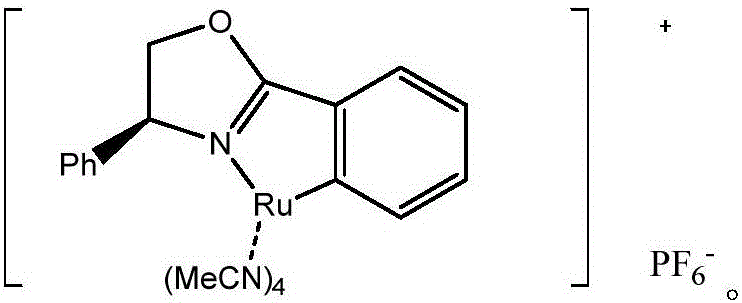
[0031] Take a dry test tube, use dioxane (0.2mL) as solvent, dissolve 1% Ru-pheox in dioxane (0.4mL), and dissolve ethyl diazoacetate (0.2mmol, 4eq) in dioxane In oxane (0.4mL), add the dissolved catalyst (0.1mL) into the reaction tube, then slowly drop ethyl diazoacetate (0.1mL) into the reaction tube, stir at room temperature for 1min, repeat the above operation 3 times , until the reaction of the raw materials is complete. The target product was obtained by column chromatography with a yield of 93%, ee: 99%. 1 HNMR (400MHz, CDCl 3 ):δ7.16(d,J=8.0Hz,1H),5.72(d,J=8.0Hz,1H),4.23-4.14(m,2H),3.51-3.47(m,1H),2.09-2.04( m,1H),1.70-1.65(m,1H),1.60(s,9H),1.47-1.41(m,1H),1.29(t,J=7.2Hz,3H); 13 CNMR (150MHz, CDCl 3 ): δ171.0, 160.4, 149.3, 143.1, 102.4, 100.1, 87.2, 61.6, 38.1, 27.6, 22.3, 15.3, 14.3. HRMS: exactmasscalcdforC 15 h 20 N 2 o 6 [M+Na] + requiresm / z347.1214, foundm / z347.1212.
Embodiment 3
[0033]
[0034] Take a dry test tube, use dioxane (0.2mL) as solvent, dissolve 2% Ru-pheox in dioxane (0.4mL), and dissolve ethyl diazoacetate (0.2mmol, 4eq) in dioxane In oxane (0.4mL), add the dissolved catalyst (0.1mL) into the reaction tube, then slowly drop ethyl diazoacetate (0.1mL) into the reaction tube, stir at room temperature for 1min, repeat the above operation 3 times , until the reaction of the raw materials is complete. The target product was obtained by column chromatography with a yield of 96%, ee: 99%. 1 HNMR (400MHz, CDCl 3 ):δ6.99(s,1H),4.21-4.16(m,2H),3.48-3.44(m,1H),2.08-2.04(m,1H),1.92(d,J=0.8Hz,3H), 1.70-1.65(m,1H),1.60(s,9H),1.47-1.42(m,1H),1.29(t,J=7.2Hz,3H); 13 CNMR (150MHz, CDCl 3 ): δ171.1, 161.4, 149.4, 147.9, 139.1, 110.9, 87.1, 61.6, 37.9, 27.6, 22.3, 15.5, 14.3, 12.5. HRMS: exactmasscalcdforC 16 h 22 N 2 o 6 [M+Na] + requiresm / z361.1370, foundm / z361.1372.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com