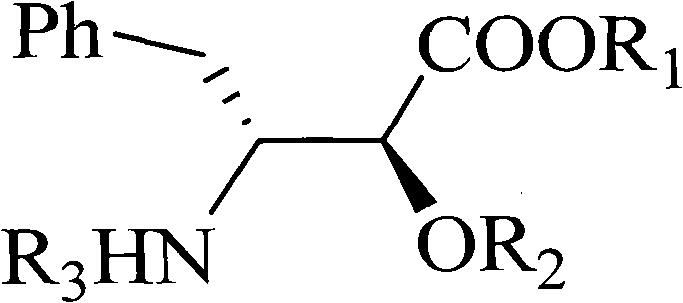
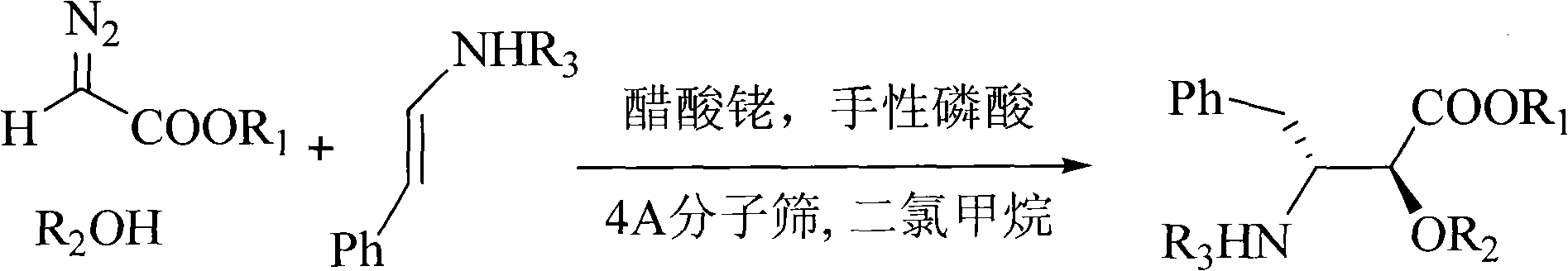
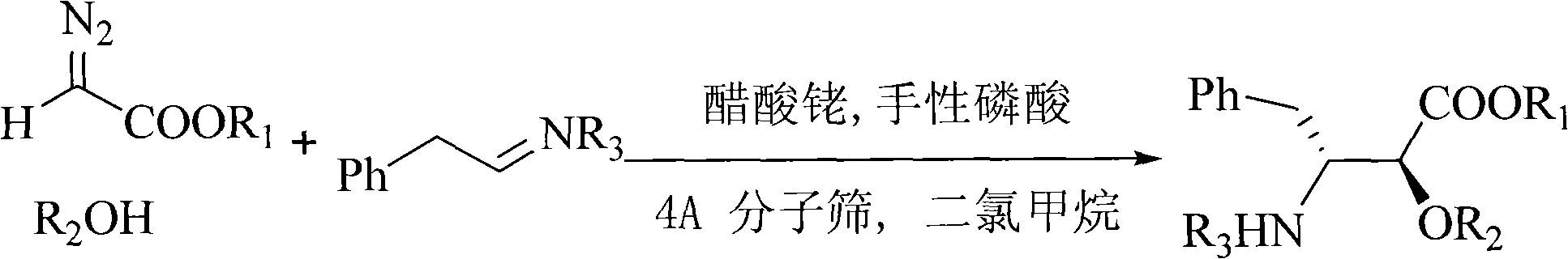
Method for synthesizing optically active alpha-hydroxyl-beta-phenmethyl-beta-amino acid derivative
A technology of optical activity and synthesis method, which is applied in the field of synthesizing optically active α-hydroxy-β-benzyl-β-amino acid derivatives, which can solve the problems of low atom economy and achieve simple and safe operation and high yield , the effect of high atomic economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] With N-tert-butoxycarbonyl styrylamine (0.30mmol), anthracenylmethanol (0.25mmol), rhodium acetate (0.005mmol), chiral phosphoric acid catalyst A (Ar=9-phenanthrenyl) shown in the structural formula on the right )(0.005mmol), Molecular sieves (0.1g) dissolved in CH 2 Cl 2 (1.5ml) to form the reaction system. Ethyl diazoacetate (0.275 mmol) was dissolved in 1 mL CH 2 Cl 2 A solution was formed in , and the solution was added dropwise to the reaction system within 1 hour with an auto-sampling pump at room temperature. After the sample injection, stir at room temperature for 1 hour, remove the solvent, and obtain the crude product; then add saturated NaHCO dropwise to the reaction system 3 Aqueous solution (0.1 ml) was used to quench the reaction. The solvent was removed by rotary evaporation under reduced pressure to obtain a crude product. The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:50~1:20) to obtain a pure product o...
Embodiment 2
[0038] N-tert-butoxycarbonyl benzyl imine (0.30mmol), anthracenylmethanol (0.25mmol), rhodium acetate (0.005mmol), the same chiral phosphoric acid A as in Example 1: Ar=9-phenanthrenyl (0.005 mmol), Molecular sieves (0.1g) dissolved in CH 2 Cl 2 (1.5ml) to form the reaction system. Ethyl diazoacetate (0.275mmol) was dissolved in 1mL CH 2 Cl 2 A solution was formed in , and the solution was added dropwise to the reaction system within 1 hour with an auto-sampling pump at room temperature. After the sample injection, stir at room temperature for 1 hour, remove the solvent, and obtain the crude product; then add saturated NaHCO dropwise to the reaction system 3 Aqueous solution (0.1 ml) was used to quench the reaction. The solvent was removed by rotary evaporation under reduced pressure to obtain a crude product. The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:50~1:20) to obtain a pure product of optically active α-hydroxy-β-benzy...
Embodiment 3
[0040] N-benzyloxycarbonyl styrylamine (0.30mmol), anthracenylmethanol (0.25mmol), rhodium acetate (0.005mmol), the same chiral phosphoric acid A as in Example 1: Ar=9-phenanthrenyl (0.005mmol ), Molecular sieve (0.1g) was dissolved in toluene (1.5ml) to form a reaction system. Methyl diazoacetate (0.275 mmol) was dissolved in 1 mL of toluene to form a solution, and the solution was added dropwise to the reaction system within 1 hour at room temperature with an automatic sample pump. After the sample injection, stir at room temperature for 1 hour, remove the solvent, and obtain the crude product; then add saturated NaHCO dropwise to the reaction system 3Aqueous solution (0.1 ml) was used to quench the reaction. The solvent was removed by rotary evaporation under reduced pressure to obtain a crude product. The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:50~1:20) to obtain a pure product of optically active α-hydroxy-β-benzyl-β-amino...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com