Synthesis method of 4-(3-butene-1-yl)-4'-alkyl-1, 1'-biphenyl liquid crystal monomer
A technology of liquid crystal monomers and synthesis methods, which is applied in the direction of liquid crystal materials, chemical instruments and methods, hydrocarbons, etc., and can solve problems such as unfavorable resource conservation and environmental protection, unfavorable industrial production requirements, and a total yield of less than 60%. , to achieve the effects of large-scale production, low cost and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
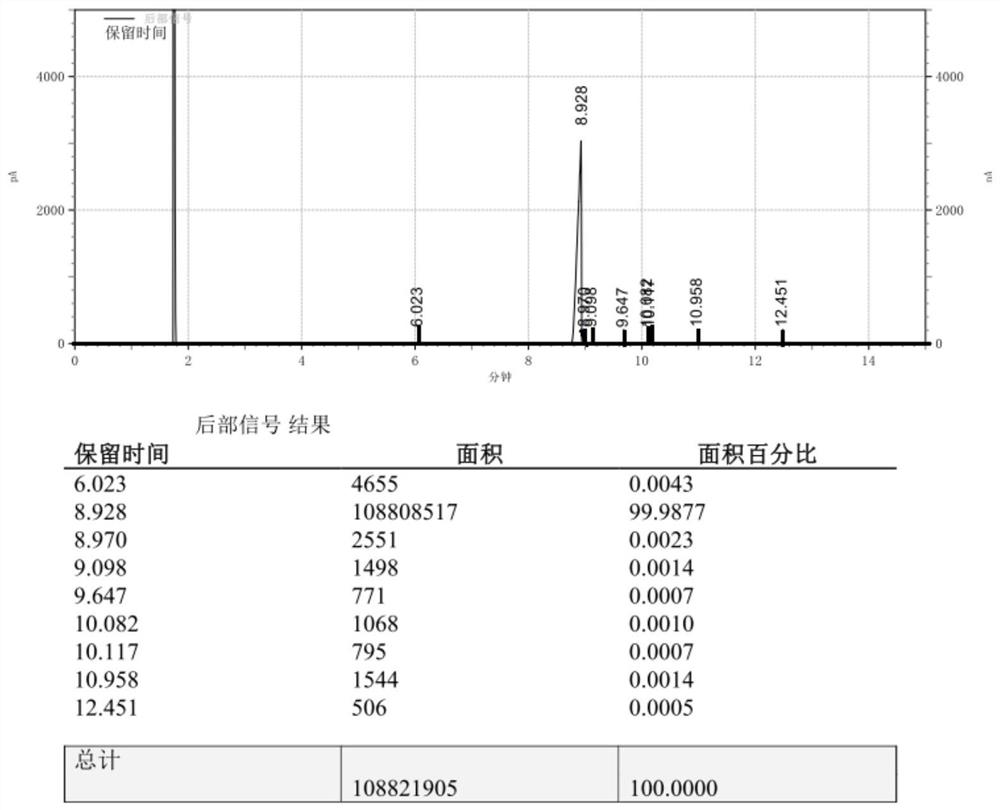
Embodiment 1
[0028] Example 1, the synthetic method of 4-(3-butene-1-methyl)-4'-methyl-1,1'-biphenyl, is as follows:
[0029]1) Under the protection of nitrogen, add 3 grains of iodine, 3.51g of magnesium chips, 6.2g of lithium chloride and 50ml of tetrahydrofuran into a 1L dry and clean three-necked flask, install a stirring device, constant pressure dropping funnel, drying device, nitrogen protection, thermometer and Heating device, dropwise add 30g of 4-bromo-4′-methyl-1,1′-biphenyl in 200ml tetrahydrofuran solution, raise the temperature to 50°C and trigger, then add the remaining 4-bromo-4′ dropwise at 25°C to 50°C -Methyl-1,1'-biphenyl solution, after the dropwise addition, the reflux reaction time is 20 to 35 minutes, and the sample is tested by GC. The content of 4-bromo-4'-methyl-1,1'-biphenyl is required to be ≤0.0050%, and the preparation Good Grignard reagent is kept at 25°C for use;
[0030] 2) Under nitrogen protection, add 1.57g Co(acac)2 and 100mL tetrahydrofuran to a 2L d...
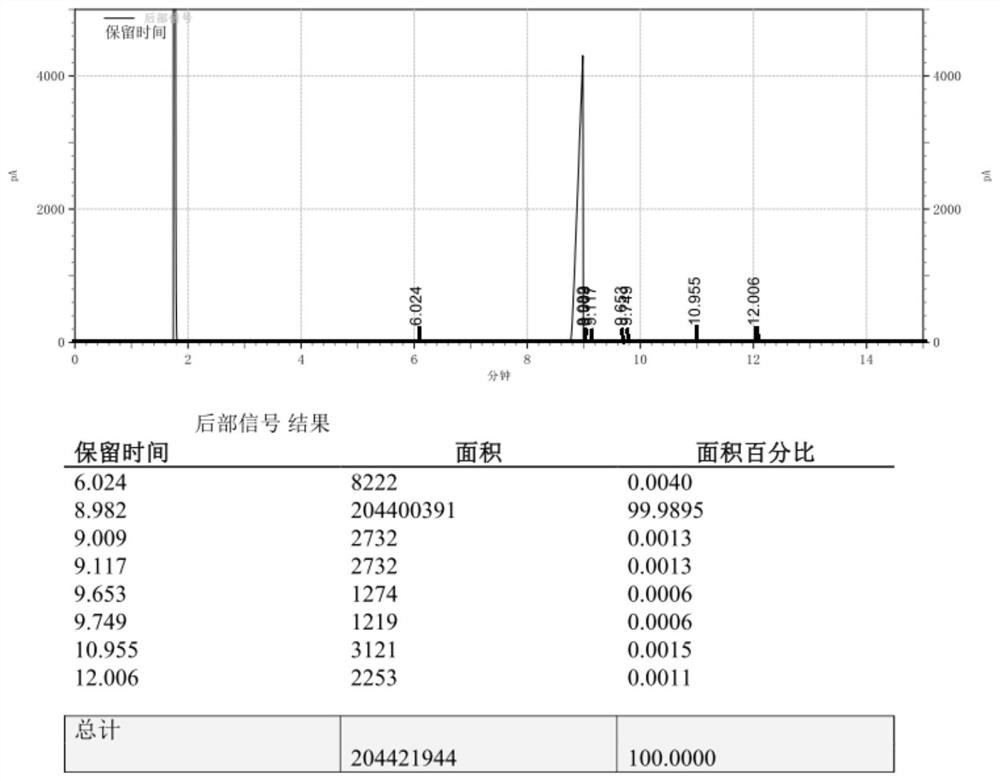
Embodiment 2
[0031] Example 2, the synthesis method of 4-(3-butene-1-methyl)-4'-methyl-1,1'-biphenyl, is as follows:
[0032] 1) Under nitrogen protection, add 3 grains of iodine, 3.51g magnesium chips, 5.74g lithium chloride and 50ml tetrahydrofuran into a 1L dry and clean three-necked flask, install a stirring device, constant pressure dropping funnel, drying device, nitrogen protection, thermometer and Heating device, dropwise add 30g of 4-bromo-4′-methyl-1,1′-biphenyl in 200ml tetrahydrofuran solution, raise the temperature to 50°C and trigger, then add the remaining 4-bromo-4′ dropwise at 25°C to 50°C -Methyl-1,1'-biphenyl solution, after the dropwise addition, the reflux reaction time is 20 to 35 minutes, and the sample is tested by GC. The content of 4-bromo-4'-methyl-1,1'-biphenyl is required to be ≤0.0050%, and the preparation It is better to keep the Grignard reagent at 25°C for use.
[0033] 2) Under nitrogen protection, add 5.58g Co(PPh)2Cl2, 100mL dioxane and 24.15g N-methylp...
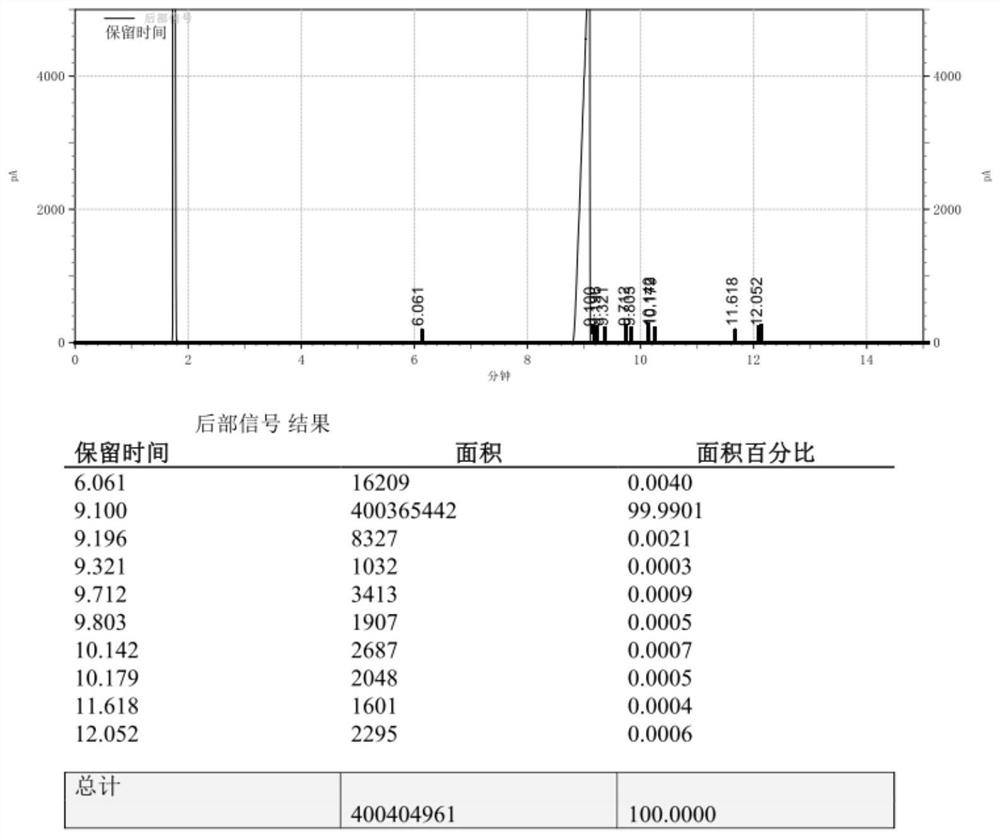
Embodiment 3
[0034] Example 3, the synthesis method of 4-(3-butene-1-methyl)-4'-methyl-1,1'-biphenyl, is as follows:
[0035] 1) Under nitrogen protection, add 3 grains of iodine, 3.51g magnesium chips, 6.98g lithium chloride and 50ml tetrahydrofuran into a 1L dry and clean three-necked flask, install a stirring device, constant pressure dropping funnel, drying device, nitrogen protection, thermometer and Heating device, dropwise add 30g of 4-bromo-4′-methyl-1,1′-biphenyl in 200ml tetrahydrofuran solution, raise the temperature to 50°C and trigger, then add the remaining 4-bromo-4′ dropwise at 25°C to 50°C -Methyl-1,1'-biphenyl solution, after the dropwise addition, the reflux reaction time is 20 to 35 minutes, and the sample is tested by GC. The content of 4-bromo-4'-methyl-1,1'-biphenyl is required to be ≤0.0050%, and the preparation It is better to keep the Grignard reagent at 25°C for use.
[0036] 2) Under nitrogen protection, add 0.79g CoCl2 and 2.62g MnBr2 and 100mL ethylene glycol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com