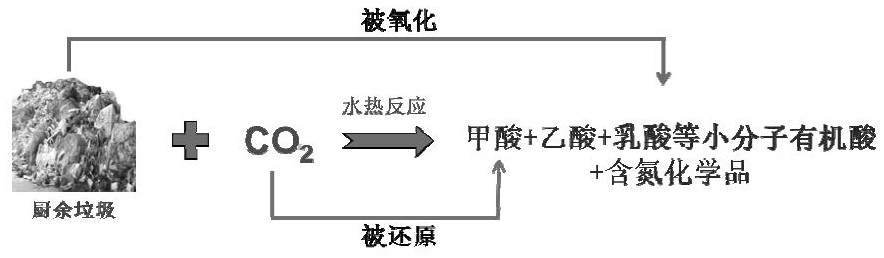
Kitchen garbage and CO2 source hydrothermal synergistic conversion method
A kitchen waste and CO2 technology, applied in chemical instruments and methods, separation methods, preparation of organic compounds, etc., to achieve the effects of no secondary pollution, simple process, and reduced process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: Starch, cellulose, protein and CO respectively 2 hydrothermal co-conversion
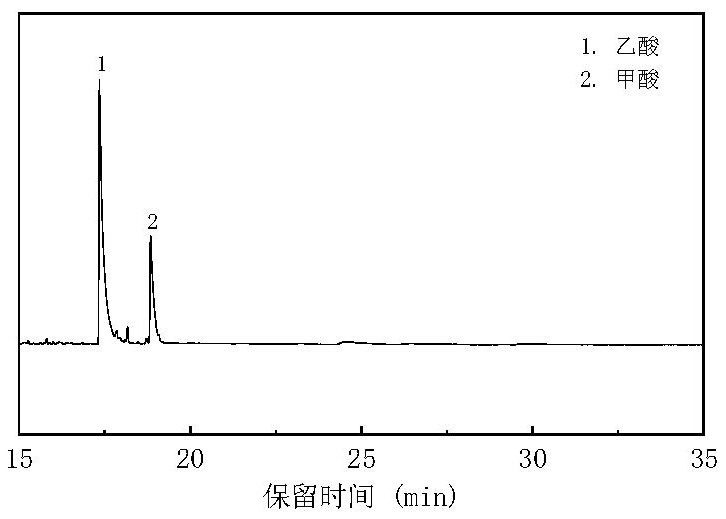
[0055] In this embodiment, the model compound of kitchen waste is taken as a representative, and it is combined with CO 2 Research on hydrothermal synergistic conversion. First, prepare starch, cellulose, and protein aqueous solutions with a water:solid ratio of 10:1, fill them into the reactor to make the filling rate 40%, and then pass CO into the reactor through the air valve. 2 , where the carbon content of kitchen waste model compounds: CO 2=1:20 molar ratio, then add a certain amount of NaOH and let stand for 5-10 hours to make the pH=9, then seal the reactor, react at 350°C for 3 hours and then cool down rapidly. The liquid phase product after the reaction is analyzed by gas chromatography (GC-MS), and the GC-MS figure of product analysis gained is as follows figure 1 , 2 , 3, carry out high-performance liquid chromatography (HPLC) analysis after liquid phase constant...
Embodiment 2
[0056] Example 2: Green vegetables and CO 2 hydrothermal co-conversion
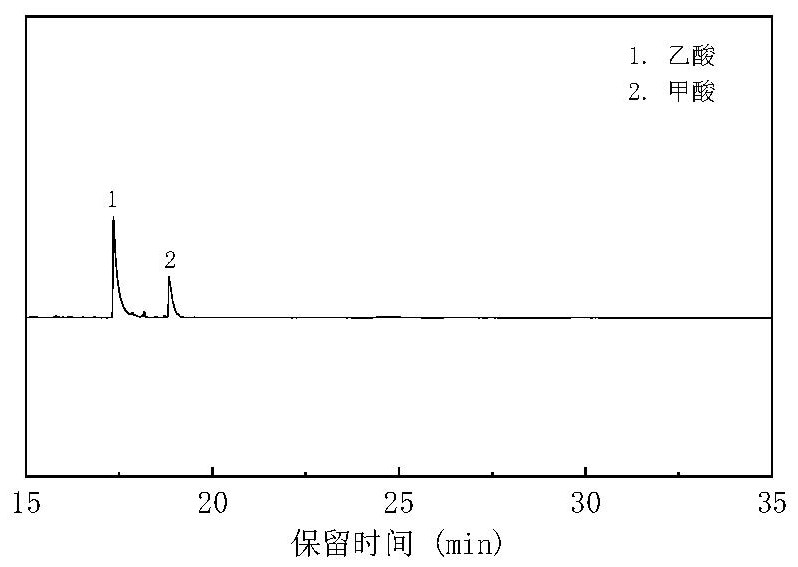
[0057] In this example, green vegetables are selected as a representative of kitchen waste to study its relationship with CO 2 Hydrothermal synergistic conversion. First, the vegetables are simply crushed, then the vegetables and water are mixed (the ratio of solid to liquid mass of the vegetables and water is 1:10) and put into the reactor, accounting for 40% of the volume of the reactor, and then CO is introduced into the reactor through the air valve. 2 , of which the carbon content of green vegetables: CO 2 =1:20 molar ratio, then add a certain amount of NaOH and let it stand for 5-10 hours to make the pH=9, then seal the reactor. After reacting at 350° C. for 3 hours, it was rapidly cooled. After the liquid phase product after the reaction was constant volume, it was analyzed by high-performance liquid chromatography (HPLC), and the gas phase was sampled and analyzed by GC-TCD. The obtained GC-TC...
Embodiment 3
[0058] Example 3: Rice and CO 2 hydrothermal co-conversion
[0059] As one of the components with higher content in kitchen waste, leftover rice is one of the typical model compounds of kitchen waste. In this example, mix cooked rice and water after pulverization (the solid-to-liquid mass ratio of rice and water is 1:10) and fill it into the reactor, accounting for 40% of the volume of the reactor, and then pass CO into the reactor through the air valve. 2 , the carbon content of leftover rice: CO 2 =1:20 molar ratio, then add a certain amount of NaOH and let it stand for 5-10 hours to make the pH=9, then seal the reactor. After reacting at 350° C. for 3 hours, it was rapidly cooled. After the liquid phase product after the reaction was constant volume, it was analyzed by high-performance liquid chromatography (HPLC), and the gas phase was sampled and analyzed by GC-TCD. The obtained GC-TCD and HPLC were quantified by the standard curve method, and the CO was calculated. 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com