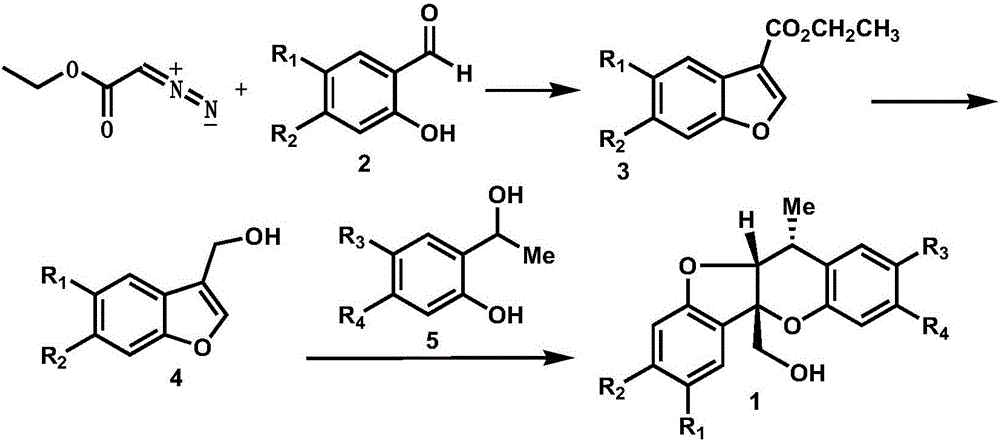
Synthetic method of paeonia veitchii lynch alcohol and structural analogue thereof
A technology with similar structure and erythromycin, which is applied in the field of natural product synthesis, can solve the problems such as inability to carry out biological activity testing and evaluation, chemical synthesis method reporting, etc., and achieves the effects of simple operation and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
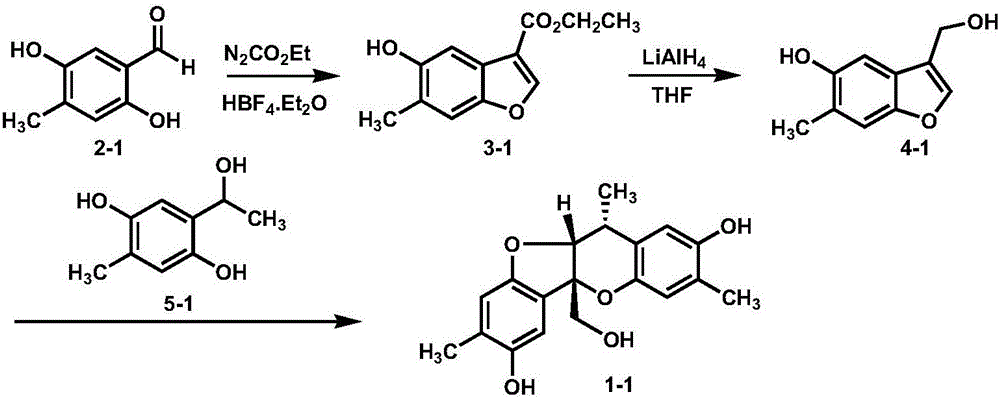
Embodiment 1
[0017]
[0018] 1. Dissolve 3.2g (21mmol) of the salicylaldehyde derivative shown in formula 2-1 in 30mL of toluene and THF in a mixed solvent with a volume ratio of 1:1, cool the system to -78°C, add 3mL (90 %, 25mmol) ethyl diazoacetate, 0.4mL (50%, 0.21mmol) tetrafluoroborate ethyl ether complex, the system was slowly warmed up to room temperature, after 12 hours of reaction, the organic solvent was removed under reduced pressure, and added to the system 3mL of concentrated sulfuric acid, stirred for 1 hour, extracted with ethyl acetate, the organic phase was dried over anhydrous magnesium sulfate, the organic solvent was removed under reduced pressure, and the residue was separated by silica gel column chromatography to obtain benzene represented by formula 3-1. And furyl ester, its productive rate is 90%, and structural characterization data is as follows: 1 HNMR (600MHz, acetone) δ: 8.37(s, 1H), 8.33(s, 1H), 7.49(s, 1H), 7.35(s, 1H), 4.35(q, J=7.1Hz, 2H), 2.34( s, 3H...
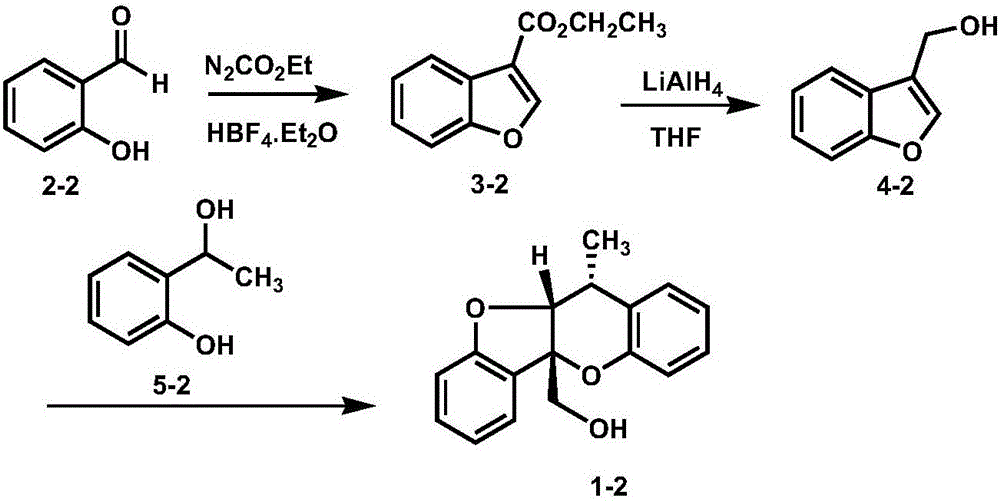
Embodiment 2
[0022]
[0023] 1. Take 2.0g (16.4mmol) of salicylaldehyde shown in formula 2-2 and dissolve it in 30mL of toluene and THF in a mixed solvent with a volume ratio of 1:1, cool the system to -78°C, add 2.6mL (90 %, 24.6mmol) ethyl diazoacetate, 0.3mL (50%, 0.16mmol) tetrafluoroborate ethyl ether complex, the system was slowly warmed up to room temperature, after 12 hours of reaction, the solvent was removed under reduced pressure, and the system was added 3mL of concentrated sulfuric acid, stirred for 1 hour, extracted with ethyl acetate, the organic phase was dried over anhydrous magnesium sulfate, the organic solvent was removed under reduced pressure, and the residue was separated by silica gel column chromatography to obtain the benzene compound shown in formula 3-2. And furyl ester, its productive rate is 92%, and structural characterization data is as follows: 1 HNMR (600MHz, CDCl 3 )δ: 8.24 (s, 1H), 8.10-8.04 (m, 1H), 7.54-7.49 (m, 1H), 7.38-7.32 (m, 2H), 4.40 (q, J=7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com