Method for preparing 4-aminobutyrate derivatives
An aminobutyrate and derivative technology, which is applied in chemical instruments and methods, cyanide reaction preparation, organic compound preparation, etc., can solve the tedious steps, environmental pollution and low yield of 4-aminobutyrate derivatives and other problems, to achieve the effect of solving the complex synthesis steps, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0013] Specific embodiment one: a method for preparing 4-aminobutyrate derivatives in this embodiment comprises the following steps:
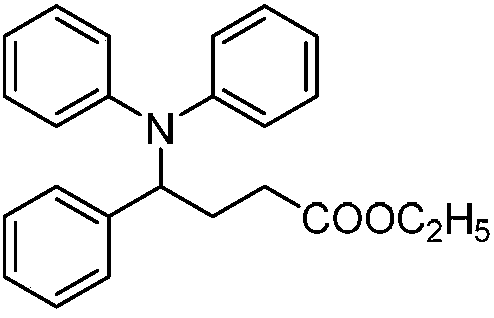
[0014] Dissolve diarylamine derivatives, olefin derivatives and ethyl diazoacetate in an organic solvent in a nitrogen atmosphere at room temperature, then add a photocatalyst and mix well, then place it under a blue LED light for light reaction, spin After steaming to remove the solvent, through silica gel column chromatography separation and purification, the resulting product is 4-aminobutyrate derivatives; the mol ratio of diarylamine derivatives, olefin derivatives, ethyl diazoacetate and photocatalyst is 1:( 2-4):(4-8):(0.1-0.5).
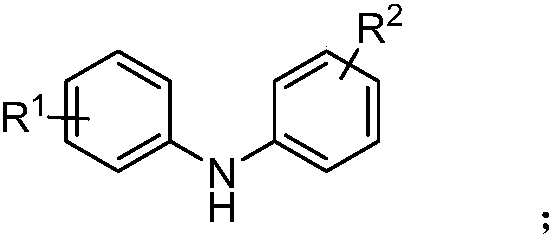
[0015] Wherein the chemical structural formula of diarylamine derivative is:
[0016] The chemical structural formula of olefin derivatives is:
[0017] The chemical structural formula of ethyl diazoacetate is:
[0018] Among them, R 1 , R 2 is alkyl, alkoxy or halogen, R 3 Is aryl, ester or alkyl.
...
specific Embodiment approach 2
[0020] Specific embodiment two: the difference between this embodiment and specific embodiment one is: olefin derivatives are styrene, β-methylstyrene, 4-methylstyrene, 4-chlorostyrene, 3-chlorostyrene or ethyl acrylate. Others are the same as the first embodiment.
specific Embodiment approach 3
[0021] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that the organic solvent is acetonitrile, tetrahydrofuran, methanol, dimethyl sulfoxide or N,N-dimethylformamide. Others are the same as those in Embodiment 1 or 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com