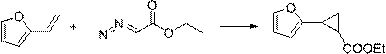Synthesis method of cyclopropane compounds
A synthetic method and compound technology, applied in the field of organic synthesis, can solve the problems of environmental pollution, low yield, long reaction time, etc., and achieve the effect of high reaction efficiency, good repeatability, and short time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
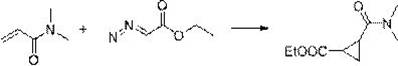
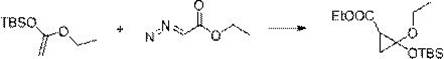
[0031] The synthesis method comprises: reacting olefin compound A and ethyl diazoacetate under the catalysis of a supported rhodium catalyst to obtain cyclopropane compounds; wherein the structural formula of olefin compound A is , R 1 , R 2 , R 3 , R 4 has the same definition as above.
[0032] The present invention catalyzes olefin compound A with supported rhodium catalyst React with ethyl diazoacetate to synthesize propane compounds. By adopting the synthesis method provided by the invention, the reaction efficiency is high, the time is short, the yield can even reach more than 90%, and the repeatability is good. Moreover, in the synthesis method, there is no need to use additives like copper halide or acetyl halide, and what is used is a supported rhodium catalyst, which has high environmental protection.
[0033] For olefin compound A For the different substituents in the present invention, whether it is H, furyl, ester group, C 1 ~C 4 Alkyl substituted ester...
Embodiment 1
[0043]
[0044] The rhodium supported catalyst (15 g) was packed into a column reactor (150 mL). Dissolve styrene (10.415g, 0.1mol) and EDA (5.705g, 0.3mol) in dichloromethane (10v), stir and clarify, then pump them into the columnar reactor at a speed of 10g / min with a back pressure of 1.0 MPa, retention time 15min, reaction temperature 25°C, outlet sampling GC. The reaction system was washed with water (10v) and separated. The organic phase was concentrated to obtain 18.2 g of reddish-brown liquid, with an isolated yield of 91%. 1 H NMR (500 MHz, Chloroform-d)δ 7.33 – 7.26 (m, 2H), 7.26 – 7.16 (m, 3H), 4.12 (qd, J = 8.0, 1.4 Hz, 2H), 2.71 (q, J = 6.9 Hz, 1H), 2.44 (q, J = 7.1 Hz, 1H), 1.88 (dt, J = 12.5, 7.0Hz, 1H), 1.77 (dt, J = 12.5, 7.0 Hz, 1H), 1.23 (t, J = 8.0 Hz, 3H).
Embodiment 2
[0046] The rhodium supported catalyst (15 g) was packed into a column reactor (150 mL). Dissolve styrene (10.415g, 0.1mol) and EDA (5.705g, 0.3mol) in ethyl acetate (10v) and stir to clarify, then pump them into the columnar reactor at a speed of 10g / min with a back pressure of 1.0 MPa, retention time 15min, reaction temperature 25°C, outlet sampling GC. The reaction system was washed with water (10v) and separated. The organic phase was concentrated to obtain 17.3 g of reddish-brown liquid, with an isolated yield of 81%. 1 H NMR (500 MHz, Chloroform-d)δ 7.33 – 7.26 (m, 2H), 7.26 – 7.16 (m, 3H), 4.12 (qd, J = 8.0, 1.4 Hz, 2H), 2.71 (q, J = 6.9 Hz, 1H), 2.44 (q, J = 7.1 Hz, 1H), 1.88 (dt, J = 12.5, 7.0Hz, 1H), 1.77 (dt, J = 12.5, 7.0 Hz, 1H), 1.23 (t, J = 8.0 Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com