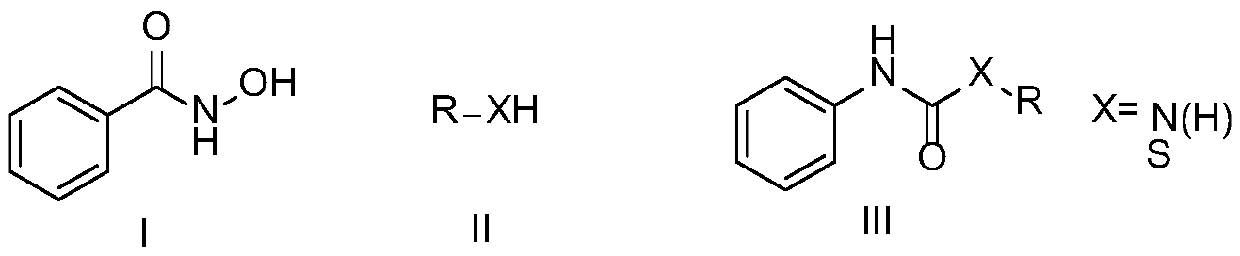
Preparation method of amide derivative
A technology of amide derivatives and substances, which is applied in the field of asymmetric urea and thiocarbamate compounds, can solve the problems of harsh reaction conditions, low conversion rate, unfavorable large-scale application, etc., and achieve simple operation process and good yield , The effect of wide substrate applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
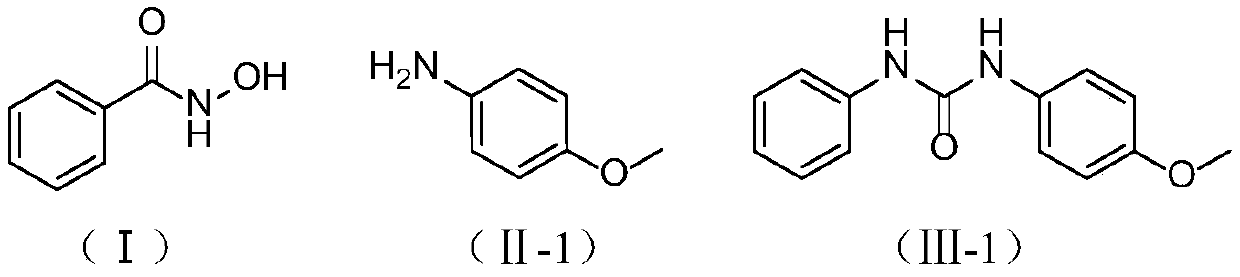
[0024] Embodiment 1: Preparation of 1-(4-methoxyphenyl)-3-phenylurea
[0025] In a 500mL single-necked flask, add 19.20g (140mmol) of benzohydroxamic acid (I), p-methoxyaniline (II-1, X=N, R 2 = 4-OCH 3 -Ph) 17.24g (140mmol), 150mL water, 45.24g (2.5eq, 350mmol) DIPEA, in SO 2 f 2 In the atmosphere, stir at 25°C for 2 hours, filter after the reaction, and wash the filter cake with 10mL of acetonitrile until white to obtain 1-(4-methoxyphenyl)-3-benzene represented by formula (Ⅲ-1). Base urea 30.87g, yield 91%.
[0026] Proton NMR spectrum: (500MHz, DMSO-d 6 )(δ,ppm):8.57(s,1H),8.46(s,1H),7.44(d,J=7.6Hz,2H),7.39–7.33(m,2H),7.27(t,J=7.9Hz ,2H),6.95(t,J=7.3Hz,1H),6.90–6.84(m,2H),3.36(s,3H).
[0027] Carbon NMR spectrum: (126MHz, DMSO-d 6 )(δ, ppm): 154.93, 153.19, 140.36, 133.17, 129.20, 122.06, 120.49, 118.54, 114.45, 55.64.
[0028]
Embodiment 2
[0029] Embodiment 2: Preparation of 1-benzyl-3-phenylurea
[0030] In a 500mL single-necked flask, add 19.20g (140mmol) of benzohydroxamic acid (I), benzylamine (II-2, X=N, R 2 =Ph-CH 2 -) 15.00g (140mmol), 150mL dichloromethane, 63.94g (3.0eq, 420mmol) DBU, in SO 2 f 2 In the atmosphere, stir at 25°C for 1 h. After the reaction, filter and rinse with 10 mL of acetonitrile until white to obtain (Ⅲ-2) 1-benzyl-3-phenylurea 26.g with a yield of 83%.
[0031] Proton NMR spectrum: (500MHz, DMSO-d 6 )(δ,ppm):8.58(s,1H),7.44–7.40(m,2H),7.33(dt,J=10.9,7.1Hz,4H),7.27–7.18(m,3H),6.90(t, J=7.3Hz, 1H), 6.64(t, J=5.7Hz, 1H), 4.31(d, J=5.9Hz, 2H).
[0032] Carbon NMR spectrum: (126MHz, DMSO-d 6 )(δ, ppm): 155.24, 140.46, 128.63, 128.29, 127.76, 127.10, 126.70, 121.07, 117.69, 42.73.
[0033]
Embodiment 3
[0034] Embodiment 3: Preparation of 3-phenyl-N-methyl-N-phenylurea
[0035] In a 500mL single-necked flask, add 19.20g (140mmol) of benzohydroxamic acid (I), N-methylaniline (II-3, X=N, R2 =N-methylanilino) 15.00g (140mmol), 150mL acetonitrile, 18.10g (3.0eq, 420mmol) Na 2 CO 3 , at SO 2 f 2 In the atmosphere, stir at 30°C for 6h, filter after the reaction, rinse with 10mL of acetonitrile until white, and you can get 3-phenyl-N-methyl-N-phenylurea 25.98 shown in formula (III-3). g, yield 82%.
[0036] Proton NMR spectrum: (500MHz, DMSO-d 6 )(δ,ppm):8.12(s,1H),7.46–7.38(m,4H),7.33(dd,J=8.4,1.1Hz,2H),7.27–7.20(m,3H),6.95(t, J=7.3Hz,1H),3.28(s,3H).
[0037] Carbon NMR spectrum: (126MHz, DMSO-d 6 )(δ, ppm): 154.74, 144.09, 140.05, 129.24, 128.26, 126.22, 125.77, 122.04, 119.90, 37.55.
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com