Synthesis method of polysubstituted pyridine derivative
A synthesis method and derivative technology, applied in the field of synthesis of multi-substituted pyridine derivatives, to achieve the effects of easy operation, avoiding the use of transition metal catalysts and toxic ligands, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
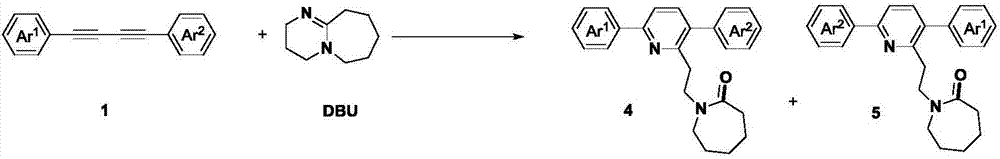
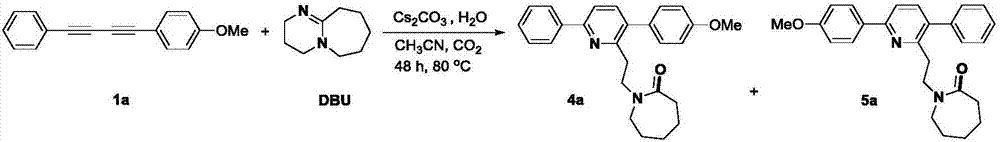
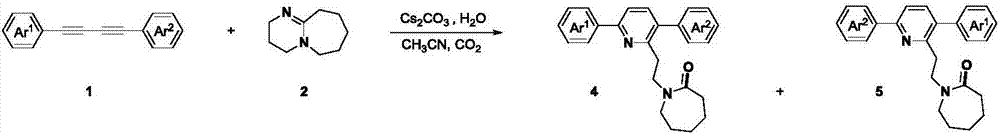
[0033] Under a carbon dioxide atmosphere, add 0.2 mmol of asymmetrically substituted 1,3-butadiyne, 0.2 mmol of cesium carbonate, 0.6 mmol of DBU, a small amount of water and 2 mL of acetonitrile into the Schlenk reaction tube in sequence, and heat and stir in IKA for 24 hours. . After the reaction, cool to room temperature, transfer the reaction solution with 20 mL of ethyl acetate, prepare samples by rotary evaporation under reduced pressure, and separate by column chromatography to obtain the target products 4a-i and 5a-i.
[0034]
[0035]
[0036] In Examples 1-9, the reactions of various 1,4-diphenylbutadiynes substituted by different substituents at the para-position of the benzene ring and DBU were studied. According to the above experiments, it can be found that the reaction has wide substrate adaptability to the substituents such as alkyl, methoxy, ethoxy, fluoro, and chloro of the benzene ring, and the corresponding target product is obtained in a higher yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com