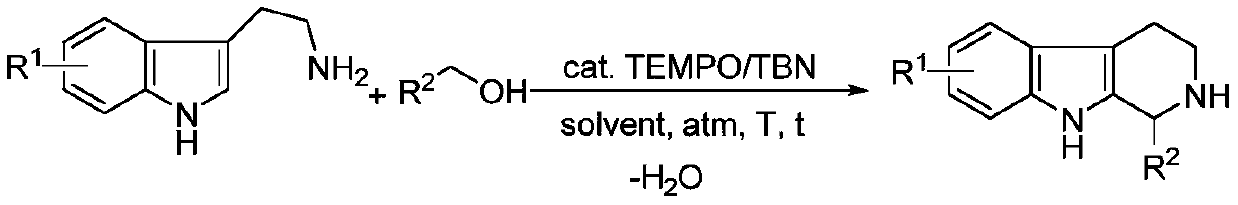
A kind of green synthetic method of tetrahydro-β-carboline heterocyclic compound
A technology for green synthesis of heterocyclic compounds, applied in chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, chemical/physical processes, etc. Toxic problems, etc., to achieve the effect of easy operation, wide application range, and low requirements for reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
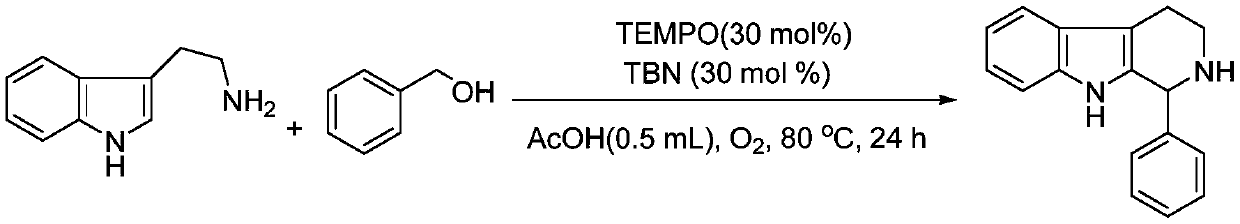
Embodiment 1
[0023] Preparation of 1-phenyl-tetrahydro-β-carboline from tryptamine and benzyl alcohol
[0024]
[0025] Add tryptamine (0.0801g, 0.5mmol), benzyl alcohol (0.0621ml, 1.2equiv.), TEMPO (0.0234g, 30mol%), TBN (0.0176ml, 30mol%) and glacial acetic acid (0.5 ml), pumping and exchanging the gas for three times, sealing the tube with oxygen, and then reacting at 80°C for 24h under stirring. After the complete reaction was monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 82%. 1 H NMR (500MHz, DMSO-d 6 ):δ10.49(br s,1H),7.43(d,J=8.0Hz,1H),7.36-7.28(m,5H),7.24(d,J=8.0Hz,1H),7.02(t,J =7.5Hz,1H),6.96(t,J=7.5Hz,1H),5.15(s,1H),3.11-3.06(m,1H),2.98-2.94(m,1H),2.79-2.67(m, 2H), 1.87(s, 1H). 13 C NMR (125.4MHz, DMSO-d 6 ): δ142.7, 135.9, 134.9, 128.5, 128.1, 127.2, 126.8, 120.5, 118.2, 117.5, 111.0, 108.2, 56.4, 40.9, 21.9.
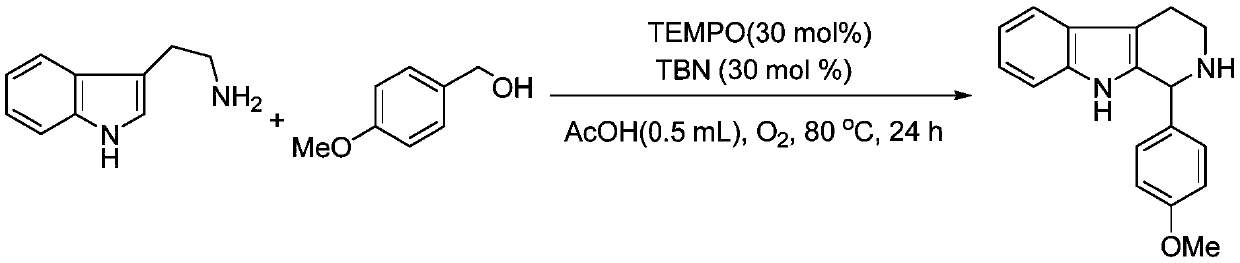
Embodiment 2
[0027] Preparation of 1-(4-methoxyphenyl)-tetrahydro-β-carboline from tryptamine and 4-methoxybenzyl alcohol
[0028]
[0029] In the tubular reactor, add tryptamine (0.0801g, 0.5mmol), 4-methoxybenzyl alcohol (0.0745ml, 1.2equiv.), TEMPO (0.0234g, 30mol%), TBN (0.0176ml, 30mol%) and glacial acetic acid (0.5ml), pumped and ventilated three times and sealed with oxygen, then reacted at 80°C for 24h under stirring. After the complete reaction was monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 94%. 1 H NMR (500MHz, DMSO-d 6 ): δ10.44(br s,1H),7.41(d,J=7.5Hz,1H),7.22(d,J=7.5Hz,1H),7.20(d,J=8.5Hz,2H),7.00( t,J=7.5Hz,1H),6.95(t,J=7.5Hz,1H),6.90(d,J=8.5Hz,2H),5.12(s,1H),3.73(s,3H),3.11- 3.09(m,1H),2.98-2.93(m,1H),2.79-2.66(m,2H),1.88(br s,1H). 13 C NMR (125.4MHz, DMSO-d 6 ): δ158.6, 135.9, 135.1, 134.5, 129.6, 126.8, 120.5, 118.1, 117.5, 113.5, 111.0, 108.0, 55.9, 55.1, 41.1, 21.9.
Embodiment 3
[0031] Preparation of 1-(4-methylphenyl)-tetrahydro-β-carboline from tryptamine and 4-methylbenzyl alcohol
[0032]
[0033] Add tryptamine (0.0801g, 0.5mmol) successively in the tubular reactor, 4-methylbenzyl alcohol (0.0733g, 1.2equiv.), TEMPO (0.0234g, 30mol%), TBN (0.0176ml, 30mol%) and Glacial acetic acid (0.5ml) was pumped and ventilated three times to seal the oxygen, and then reacted at 80°C for 24h under stirring. After the complete reaction was monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 89%. 1 H NMR (500MHz, DMSO-d 6 ):δ10.37(br s,1H),7.40(d,J=7.5Hz,1H),7.21(d,J=7.5Hz,1H),7.17-7.12(m,4H),6.99(t,J =7.5Hz,1H),6.94(t,J=7.5Hz,1H),5.04(s,1H),3.09-3.04(m,1H),2.94-2.89(m,1H),2.73-2.63(m, 2H), 2.28(s, 1H). 13 C NMR (125.4MHz, DMSO-d 6 ): δ140.1, 136.2, 135.9, 135.5, 128.6, 128.3, 126.8, 120.4, 118.1, 117.4, 110.9, 108.1, 56.3, 41.3, 22.2, 20.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com