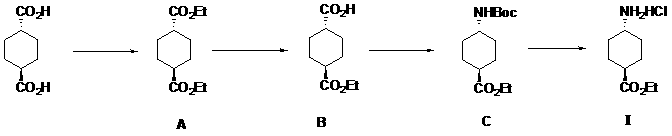
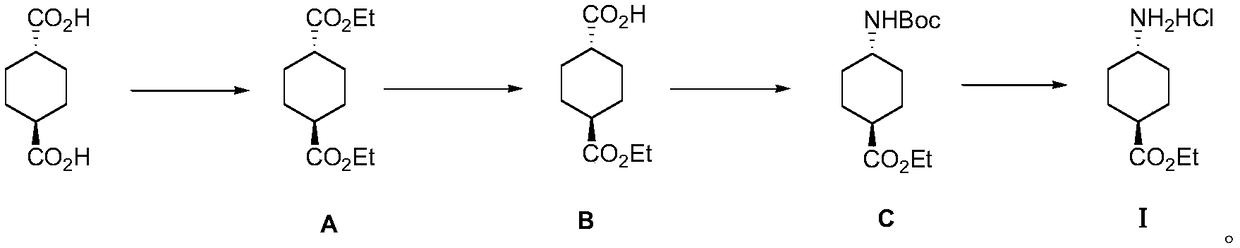
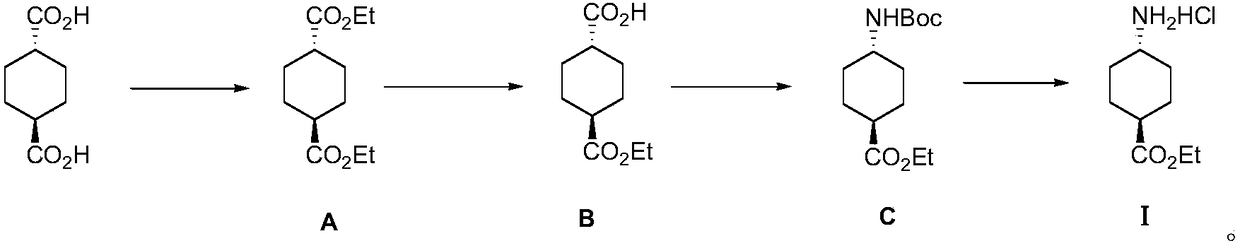
Method for preparing ethyl trans-4-amino-cyclohexanecarboxylate hydrochloride
A technology of ethyl cyclohexanecarboxylate hydrochloride and cyclohexanedicarboxylic acid, which is applied in the preparation of carbamic acid derivatives, the preparation of carboxylate, and the preparation of carboxylate/lactone, etc. Low, corroded equipment and other problems, to achieve the effect of solving the problem of magnified risk, easy operation and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0033] S1: Add 2.4 liters of ethanol and 400 grams of trans-1,4-cyclohexanedicarboxylic acid into a 5-liter round-bottomed flask, stir and mix thoroughly, then add 12 milliliters of concentrated sulfuric acid, heat up to reflux state and react for about 20 hours, point Plate detection raw material reaction is complete, cooled down to room temperature, concentrated, dissolved with 500 ml of ethyl acetate, washed to neutral, dried and concentrated to obtain 531 g of diethyl trans-1,4-cyclohexanedicarboxylate as light yellow Oily liquid, yield 100%;
[0034] S2: In a 5-liter three-necked round-bottomed flask, dissolve 530 grams of the light yellow oily liquid obtained in the previous step in 3 liters of a mixed solvent of anhydrous toluene and absolute ethanol (volume ratio 1:2), and stir under room temperature. Add 93 grams of sodium hydroxide in absolute ethanol to obtain a white suspension, continue to stir until the pH value does not change, point the plate to detect that the...
experiment example 2
[0038] S1: Add 2.5 liters of ethanol and 450 grams of trans-1,4-cyclohexanedicarboxylic acid into a 5-liter round-bottomed flask, stir and mix thoroughly, then add 10 milliliters of concentrated sulfuric acid, heat up to reflux state and react for about 20 hours, point Plate detection raw material reaction is complete, cooled down to room temperature, concentrated, dissolved with 500 ml of ethyl acetate, washed with water until neutral, dried and concentrated to obtain 596 g of trans-1,4-diethyl cyclohexanedicarboxylate as light yellow Oily liquid, yield 100%;
[0039] S2: In a 5-liter three-necked round-bottomed flask, dissolve 596 grams of the light yellow oily liquid obtained in the previous step in a mixed solvent of 3.1 liters of anhydrous toluene and absolute ethanol (volume ratio 1:3), and drop under stirring at room temperature Add 104 grams of sodium hydroxide in absolute ethanol to obtain a white suspension, continue to stir until the pH no longer changes, point the ...
experiment example 3
[0043] S1: Add 10 liters of ethanol and 1720 grams of trans-1,4-cyclohexanedicarboxylic acid into a 20 liters reactor, stir and mix thoroughly, and then add 100 milliliters of concentrated sulfuric acid. Raise the temperature to a reflux state and react for about 20 hours. The reaction of the raw materials is detected by pointing a plate, and the reaction is cooled down to room temperature. Concentrate, dissolve with 5 liters of ethyl acetate, wash with water until neutral, dry and concentrate to obtain 2280 g of diethyl trans-1,4-cyclohexanedicarboxylate as light yellow oily liquid with a yield of 100%.
[0044] S2: In a 20-liter reactor, dissolve 2280 grams of the light yellow oily liquid obtained in the previous step in a mixed solvent of 15 liters of anhydrous toluene and absolute ethanol (volume ratio 1:1), and add 400 grams of it dropwise under stirring at room temperature Anhydrous ethanol solution of sodium hydroxide, to obtain a white suspension, continue to stir unti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com