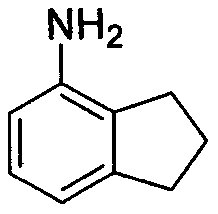
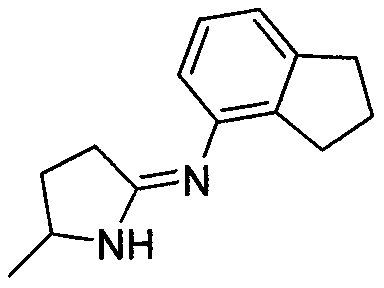
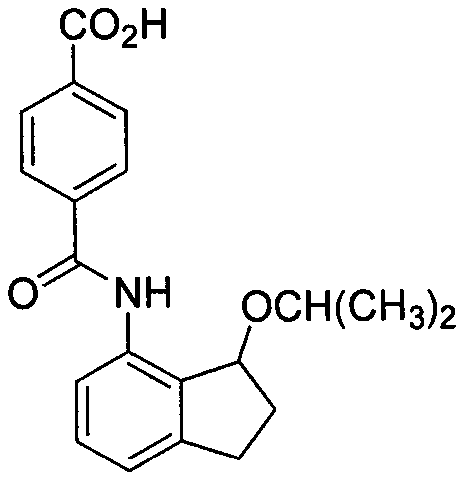
New preparation method of 4-aminoindan compound
A technology of aminoindane and compound, which is applied in the field of drug synthesis, can solve problems such as low yield of nitration reaction, poor regioselectivity, and difficulty in separation and purification of final products, and achieve the effects of avoiding separation difficulties, high yield, and good industrialization prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of Intermediate 2:
[0028] Dissolve 1.5g (10mmol) of compound 1 (o-carboxybenzaldehyde) in 10mL of thionyl chloride, slowly add 10.0mL of anhydrous methanol dropwise to the system in an ice bath, stir for 10min after the dropwise addition, and then move to room temperature After stirring for about 0.5 h, TLC monitored that the reaction was complete. After the solvent was removed under reduced pressure, the solvent was quickly purified on a silica gel column to obtain 1.31 g of a colorless oily liquid 2 with a yield of 82%.
Embodiment 2
[0030] Preparation of intermediate 3:
[0031] Preparation of triethylamine formic acid solution: Take a 25.0mL round bottom flask and add 0.98g (3.5eq) of formic acid, add 0.91g (1.4eq) of triethylamine dropwise under ice-bath conditions, and stir for ten minutes after the addition is complete.
[0032] Add 3.0mL DMF to a 50.0mL round-bottom flask to dissolve 1.0g of compound 2, then add 0.97g (1.1eq) of McFarlandic acid and stir, the system is yellow and transparent, then add dropwise the triethylamine formic acid solution prepared above, after the addition is complete Move to an oil bath at 100°C and heat to react for 6 hours. TLC monitors that the reaction of the raw materials is complete, and proceed to the reaction treatment: use an oil pump to dry up the solvent, add water to dilute, and then use 1M dilute hydrochloric acid to adjust the pH of the system to 2. At this time, a yellow solid will precipitate and be filtered by suction , it was subjected to flash silica gel...
Embodiment 3
[0034] Preparation of Intermediate 4:
[0035] Add 20.0mL 1,2-dichloroethane to a 50.0mL round-bottom flask to dissolve 1.5g of compound 3, then add 1.7g (2.0eq) of thionyl chloride dropwise, then heat the reaction in an oil bath at 80°C for 3h, then concentrate under reduced pressure to remove the solvent , and the obtained oil was dissolved in 5.0 mL 1,2-dichloroethane for later use. In addition, take a 50.0mL round bottom flask and add 20.0mL 1,2-dichloroethane, then add 1.9g (2.0eq) of aluminum trichloride and stir, the system is cooled in an ice bath, and then the acid chloride solution prepared above is added dropwise, the system Turn into a brown transparent oil bath, then heat the reaction at 80°C for 6h, monitor the reaction by TLC, and carry out post-processing: quench the reaction with saturated sodium bicarbonate solution, then filter the precipitated solid with diatomaceous earth, and concentrate the filtrate under reduced pressure to remove the solvent. Add wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com