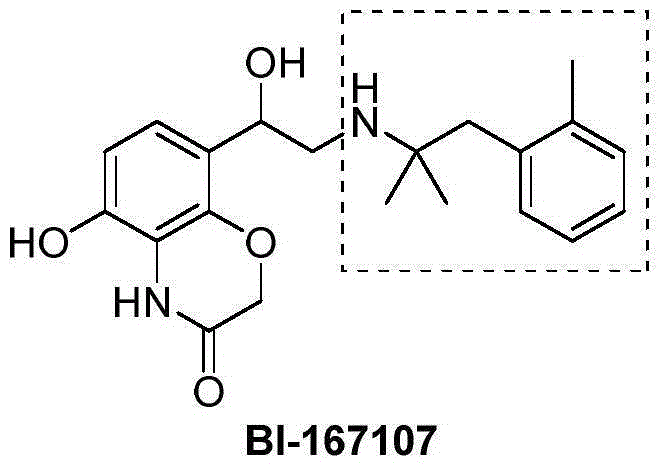
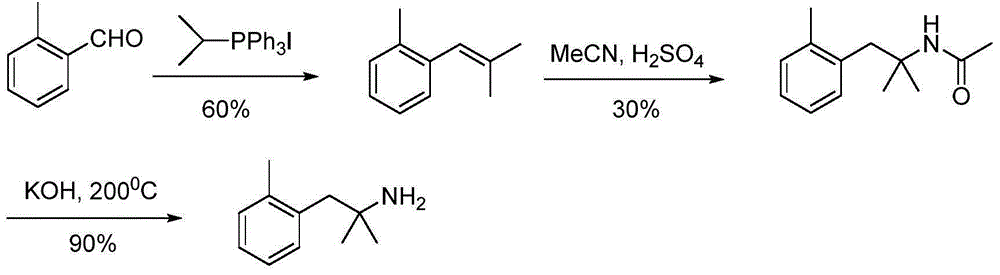
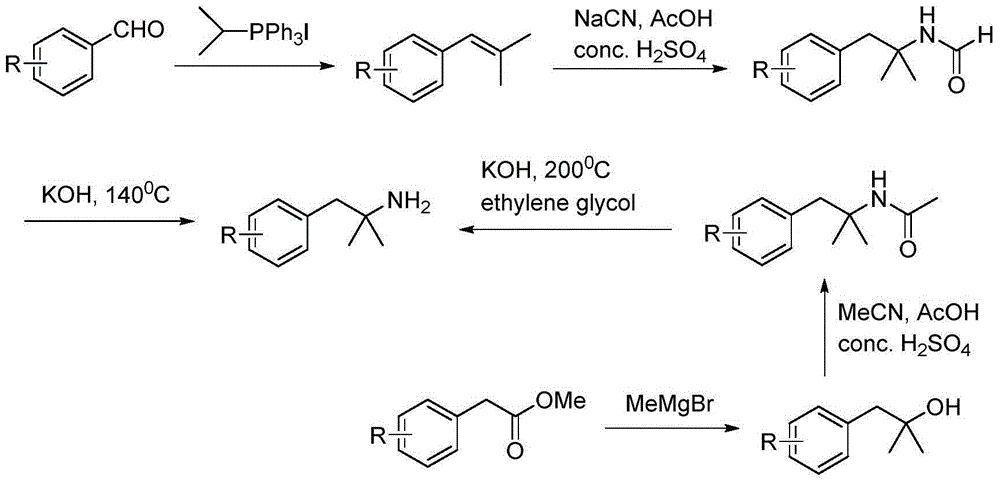
Method for preparing 2-methyl-1-substituted phenyl-2-propyl amine compound
A compound and phenyl technology, applied in the field of pharmaceutical synthesis, can solve problems such as low yield, and achieve the effects of simple operation, cheap and easy-to-obtain raw materials, and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of 2-methyl-1-(2-methylphenyl)-2-propanamine (5a; R=2-Me)
[0040] Step 1: Preparation of 2-methyl-1-(2-methylphenyl)-2-butyronitrile (2a; R=2-Me)
Embodiment 1
[0041] Example 1: Dissolve diisopropylamine (1.88mL, 13.4mmol, 1.1eq) in 10mL of tetrahydrofuran, ice-bath to -78°C, and 2 Under protection, n-butyllithium (2.8mL, 7.0mmol, 1.1eq) was added dropwise, and after stirring for 30min, light yellow LDA was prepared. Isobutyronitrile (2.39mL, 26.6mmol, 1.1eq) was added dropwise to the reaction, stirred for 1 hour, then 2-methylbenzyl chloride (1.5g, 10.6mmol) was added, and the reaction was stopped after stirring for 25min. Add 10mL of 10% hydrochloric acid and 30ml of water to the mixture to quench the reaction, extract 3 times with 20mL of ethyl acetate, wash 3 times with 15mL of water and 3 times with 15mL of saturated brine, pour the organic phase into Add an appropriate amount of anhydrous sodium sulfate to a clean Erlenmeyer flask and dry it for half an hour. After purification by column chromatography, a pale yellow liquid was obtained with a yield of 98%. 1 HNMR (300MHz, CDCl 3 ): δ7.30-7.16 (m, 4H), 2.88 (s, 2H), 2.38 (s,...
Embodiment 2
[0042] Example 2: Other conditions were the same as Example 1, except that the molar ratio of 2-methylbenzyl chloride to isobutyronitrile was changed to 1:3, and the yield of the obtained product was 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com