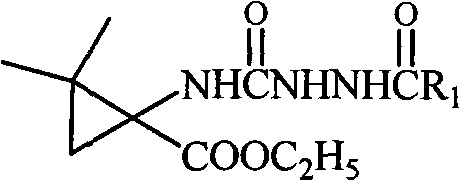N-3-aramid-5-cyclopropane spiro hydantoin derivative, preparation method and application thereof
A technology of spirocyclic hydantoin and cyclopropane, which is applied in the field of hydantoin derivatives and their preparation, can solve the problems of cognitive dysfunction, severe allergic reactions, adverse reactions and the like, and achieves simple preparation method and high yield. high rate effect
Inactive Publication Date: 2010-10-20
SYNCOM CHINA CO LTD WUHAN
View PDF2 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Although the new antiepileptic drugs have partially improved the shortcomings of some antiepileptic drugs, most of the new drugs can still be accompanied by some special adverse reactions, such as cognitive dysfunction, acute eye symptoms, severe allergic reactions, etc.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 20
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
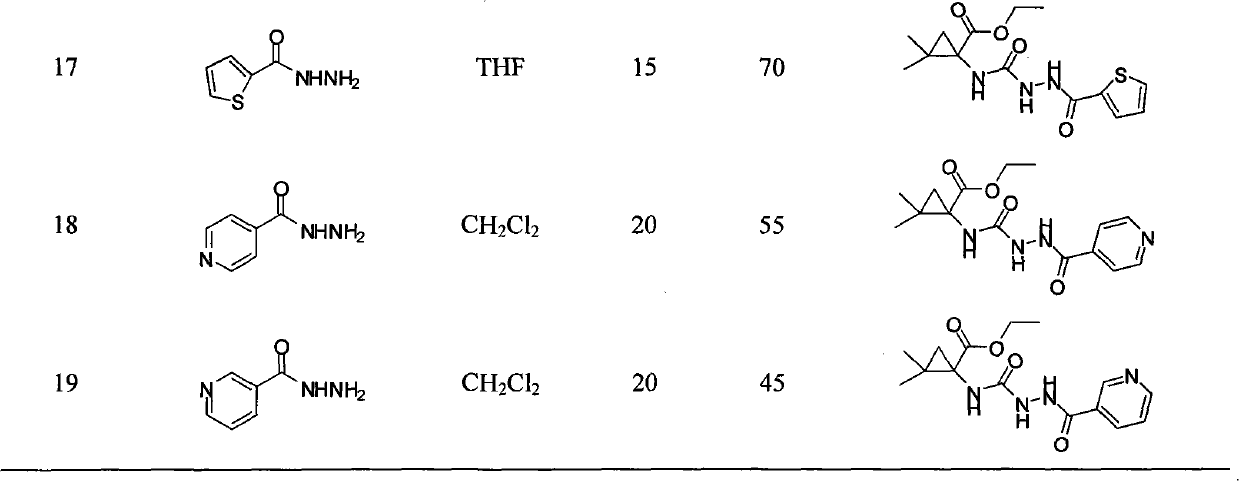
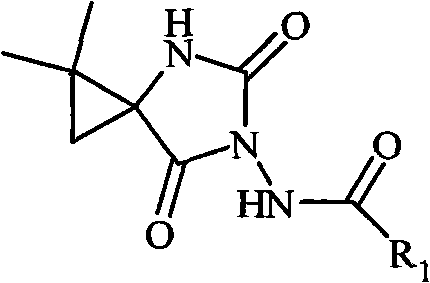
The invention relates to an N-3-aramid-5-cyclopropane spiro hydantoin derivative which has a structural formula described in the specification, wherein R1 is phenyl, substituted phenyl and heterocycle aryl. A preparation method comprises the steps of: reacting 1-carboxyl-2,2-dimethylcyclopropane carboxylic ethyl ester with ethyl chloroformate to generate 1-acid azide-2,2-dimethylcyclopropane carboxylic ethyl ester under the action of NaN3; carrying out Curtius rearrangement on the 1-acid azide-2,2-dimethylcyclopropane carboxylic ethyl ester to generate isocyanate, reacting the isocyanate with arylacethydrazide to obtain N'-aramid substituted carbamido cyclopropane; and then carrying out cyclization on the N'-aramid substituted carbamido cyclopropane under the alkaling condition to generate the N-3-aramid-5-cyclopropane spiro hydantoin derivative. The compound has relatively good convulsions resisting activity, simple preparation method and higher yield.
Description
technical field The invention relates to a hydantoin derivative, a preparation method and application thereof. Background technique Hydantoin, also known as hydantoin, has attracted widespread attention since its discovery in 1861. Some hydantoin derivatives have unique pharmacological activities and are widely used in medicine. Among them, a representative one is phenytoin sodium, chemical name 5-ethyl-5-phenylhydantoin, which is a common drug for treating epilepsy. However, it has been clinically found that long-term use of phenytoin sodium can cause gingival hyperplasia, with large side effects. Since the 1980s, with the in-depth study of the pathogenesis of epilepsy, the mechanism of action of antiepileptic drugs has been clarified, and some new antiepileptic drugs have been designed on this basis. At present, the new antiepileptic drugs that have been approved for clinical application abroad are: zonisamide, oxcarbazepine, lamotrigine, felbamate, vigabatrin, gabapen...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D235/02C07D405/12C07D409/12C07D401/12A61K31/4184A61K31/4439A61P25/08
Inventor 胡先明贺贤然
Owner SYNCOM CHINA CO LTD WUHAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com