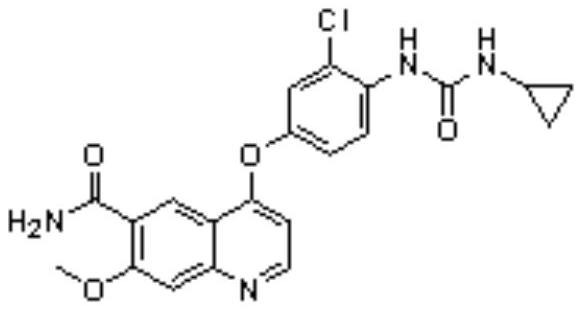
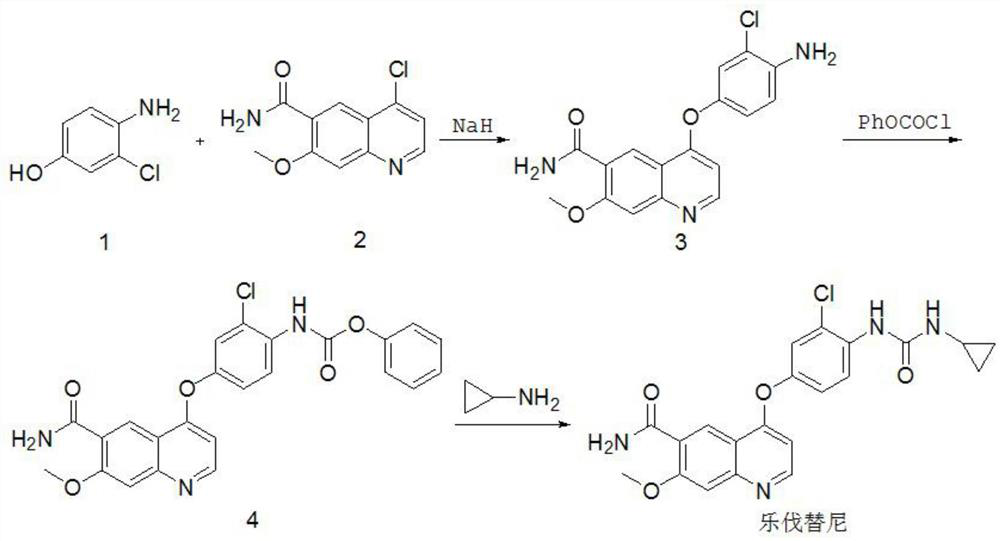
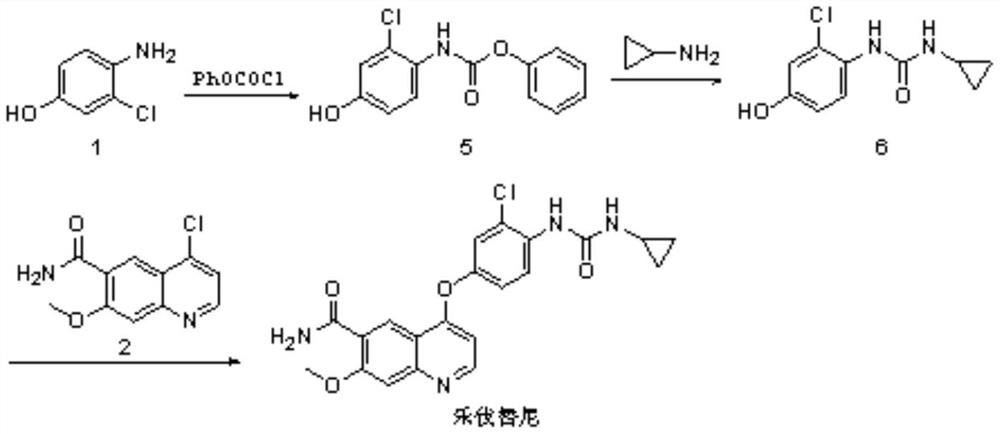
Synthesis method of lenvatinib
A synthesis method and technology of lenvatinib, which are applied in the field of medicinal chemical synthesis, can solve the problems of low intermediate purity, low yield, danger and the like, and achieve the effects of simple reaction steps, simple and easy-to-obtain raw materials, and low production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Dissolve methyl 2-chloro-4-hydroxybenzoate in dimethyl sulfoxide, add potassium carbonate and 4-chloro-7-methoxyquinoline-6-amide at room temperature, stir and heat to reflux for 10 hours . The molar ratio of methyl 2-chloro-4-hydroxybenzoate, 4-chloro-7-methoxyquinoline-6-amide and base is 1:0.5:1.1. After the reaction was completed, naturally cool to room temperature, concentrate under reduced pressure, pour the residue into ice water, filter, wash the filter cake with water, and recrystallize with isopropanol to obtain 4-(3-chloro-4-methoxycarbonylphenoxy) -7-methoxy-6-quinolinecarboxamide, yield 83.3%.
[0052] Dissolve 4-(3-chloro-4-methoxycarbonylphenoxy)-7-methoxy-6-quinolinecarboxamide in methanol, add 1 mol / L sodium hydroxide aqueous solution, and stir at room temperature for 18 hours. The molar ratio of 4-[3-chloro-4-methoxycarbonylphenoxy]-7-methoxy-6-quinolinecarboxamide to base is 1:2.0; 4-[3-chloro-4-methoxy The weight ratio of carbonylphenoxy]-7-methox...
Embodiment 2
[0055] Dissolve methyl 2-chloro-4-hydroxybenzoate in N,N-dimethylformamide, add sodium carbonate and 4-chloro-7-methoxyquinoline-6-amide at room temperature, stir and heat to Reflux for 10 hours. The molar ratio of methyl 2-chloro-4-hydroxybenzoate, 4-chloro-7-methoxyquinoline-6-amide and base is 1:0.9:1.5. After the reaction was completed, naturally cool to room temperature, concentrate under reduced pressure, pour the residue into ice water, filter, wash the filter cake with water, and recrystallize with isopropanol to obtain 4-(3-chloro-4-methoxycarbonylphenoxy) -7-methoxy-6-quinoline carboxamide, yield 83.9%.
[0056] Dissolve 4-(3-chloro-4-methoxycarbonylphenoxy)-7-methoxy-6-quinolinecarboxamide in ethanol, add 1 mol / L potassium hydroxide aqueous solution, and stir at room temperature for 18 hours. The molar ratio of 4-[3-chloro-4-methoxycarbonylphenoxy]-7-methoxy-6-quinolinecarboxamide to base is 1:3.0; 4-[3-chloro-4-methoxy The weight ratio of carbonylphenoxy]-7-meth...
Embodiment 3
[0059] Dissolve methyl 2-chloro-4-hydroxybenzoate in acetonitrile, add potassium hydroxide and 4-chloro-7-methoxyquinoline-6-amide at room temperature, stir and heat to reflux for 10 hours. The molar ratio of 2-chloro-4-hydroxybenzoic acid methyl ester, 4-chloro-7-methoxyquinoline-6-amide and base is 1:1.5:0.9. After the reaction was completed, naturally cool to room temperature, concentrate under reduced pressure, pour the residue into ice water, filter, wash the filter cake with water, and recrystallize with isopropanol to obtain 4-(3-chloro-4-methoxycarbonylphenoxy) -7-methoxy-6-quinoline carboxamide, yield 81.5%.
[0060] Dissolve 4-(3-chloro-4-methoxycarbonylphenoxy)-7-methoxy-6-quinolinecarboxamide in tetrahydrofuran, add 1mol / L sodium hydroxide aqueous solution, and stir at room temperature for 18 hours. The molar ratio of 4-[3-chloro-4-methoxycarbonylphenoxy]-7-methoxy-6-quinolinecarboxamide to base is 1:2.5; 4-[3-chloro-4-methoxy The weight ratio of carbonylphenoxy]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com