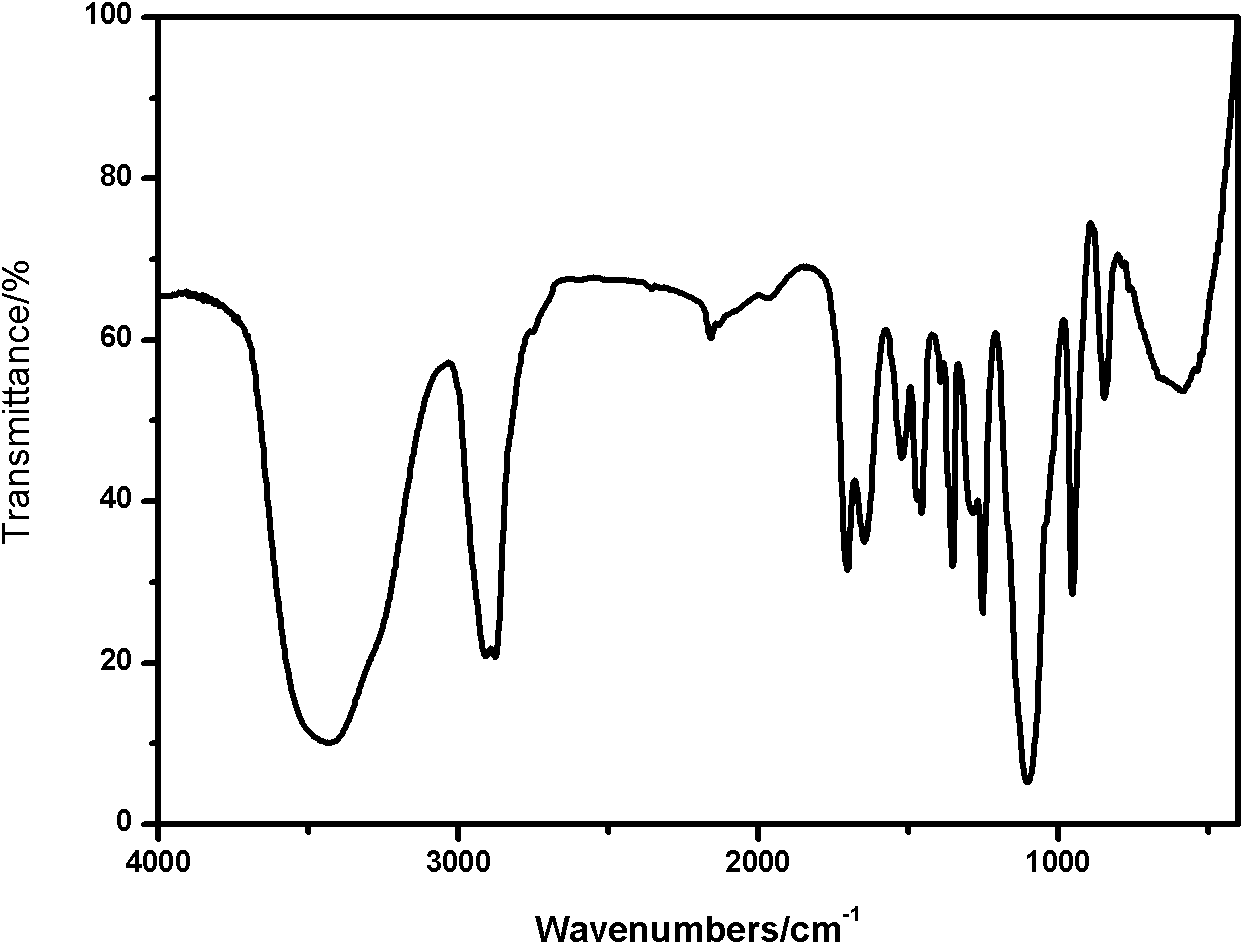
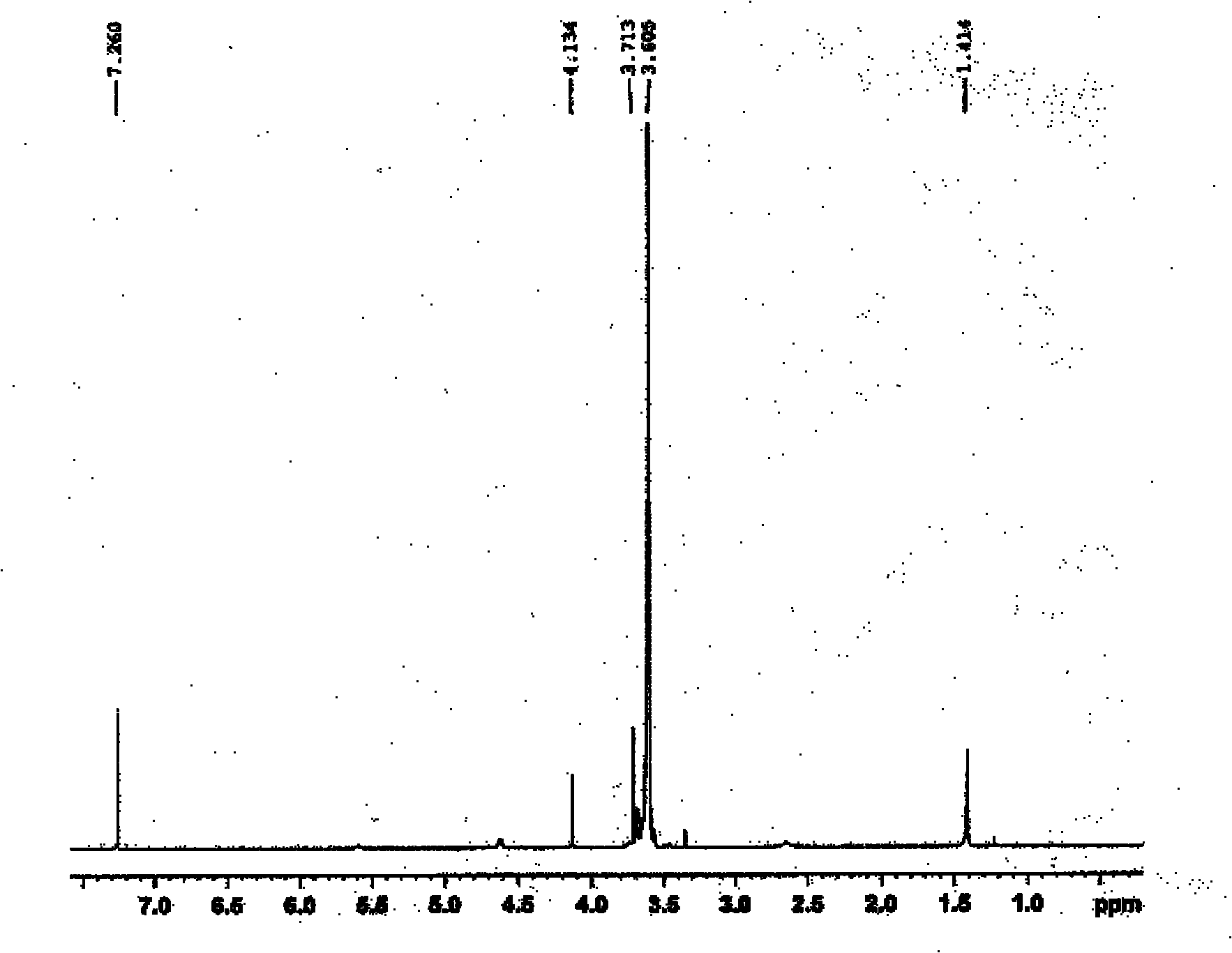
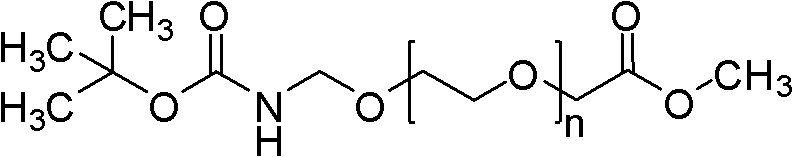
Polyethylene glycol omega-amino acid and preparation method thereof
A polyethylene glycol and amino acid technology, which is applied in the preparation of carbamic acid derivatives, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of complex separation and low yield of monochlorination, and avoid complicated operations, The effect of novel structure and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Dissolve 6 g of polyethylene glycol (PEG, molecular weight 1000), 0.12 g of sodium bromide, and 0.01 g of TEMPO into 20 mL of water, and stir magnetically under an ice bath. Measure 26mL of sodium hypochlorite solution (available chlorine > 10%) and add 4mol·L dropwise -1 Hydrochloric acid adjusts the pH to around 10. Keep the reaction temperature and pH constant, and react for 1-2 hours. After the reaction was completed, 3 mL of ethanol was added to terminate the reaction. Drop into 4mol·L -1 Adjust the pH value to less than 2 with hydrochloric acid, add ether and wash 3 times. Dichloromethane was added for extraction, and the oil phase was rotary evaporated, then precipitated with ether, and dried in vacuum to obtain 5.93 g of α, ω-dicarboxypolyethylene glycol, with a yield of 96.1%.
[0031] 2g of dichlorotrityl chloride resin (crosslinking degree 1% DVB, substitution degree 0.95mmol·g -1 ) swelling in dichloromethane for 10min. 5 g of synthetic α,ω-dicarboxypo...
Embodiment 2
[0036] Dissolve 10 g of polyethylene glycol (PEG, molecular weight 2000), 100 mg of sodium bromide, and 8 mg of TEMPO into 20 mL, and stir magnetically under an ice bath. Measure 22mL of sodium hypochlorite solution (available chlorine > 10%) and add 4mol·L dropwise -1 Hydrochloric acid to adjust the pH to about 10. Keep the reaction temperature and pH constant, and react for 1-2h. After the reaction was completed, 3 mL of ethanol was added to terminate the reaction. Drop into 4mol·L -1 Adjust the pH value to less than 2 with hydrochloric acid, add ether and wash 5 times. Dichloromethane was added for extraction, and the oil phase was rotary evaporated, then precipitated with ether, and dried in vacuo to obtain 10.1 g of α,ω-dicarboxypolyethylene glycol with a yield of 99.2%.
[0037] Swell 2 g of dichlorotrityl chloride resin (crosslinking degree 1% DVB, substitution degree 0.95 mmol / g) in dichloromethane for 10 min. 10 g of synthesized α,ω-dicarboxypolyethylene glycol w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com