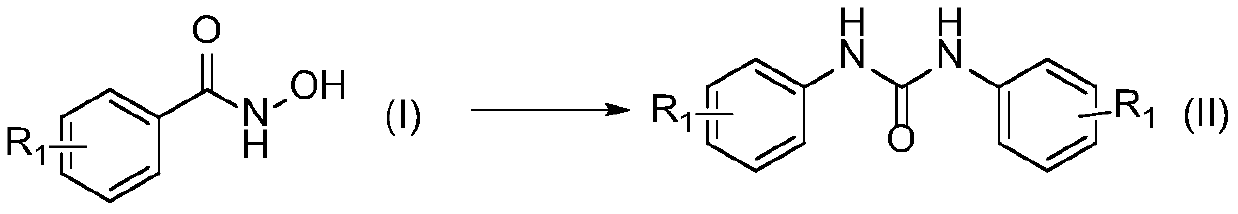
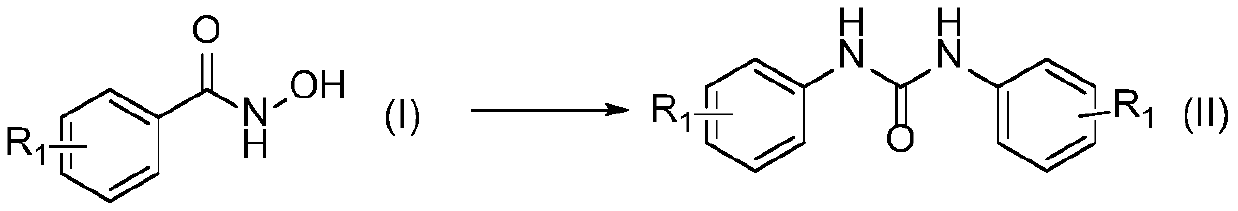
Preparation method of symmetric urea compound
A compound and urea technology, applied in the field of preparation of symmetrical urea compounds, can solve the problems of unfriendly environment, unfavorable large-scale application, etc., and achieve the effects of good yield, wide substrate applicability and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
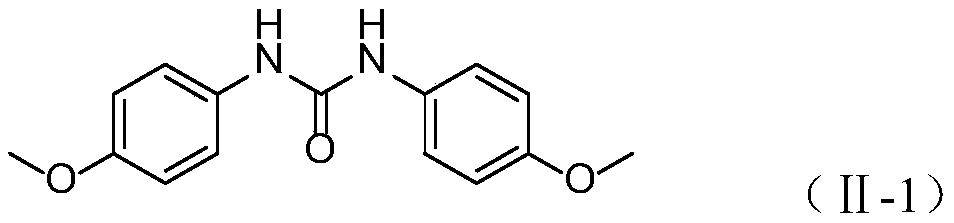
[0025] Embodiment 1: the preparation of 1,3-bis (4-methoxyphenyl) urea
[0026] In a 500mL single-necked flask, add 4-methylphenylhydroxamic acid (formula Ⅰ-1, R 1 = 4-OCH 3 ) 23.40g (140mmol), 150mL water, 45.24g (2.5eq, 350mmol) DIPEA, in SO 2 f 2 In the atmosphere, stirring at 25°C for 2h, after the reaction, the reaction solution was filtered, and the filter cake was rinsed with acetonitrile to white to obtain 17.35g of 1,3-bis(4-methoxyphenyl)urea, the yield 91%.
[0027] Proton NMR spectrum: (500MHz, DMSO-d 6 ) (δ, ppm): 8.36 (s, 2H), 7.33 (d, J = 9.0Hz, 4H), 6.85 (d, J = 9.0Hz, 4H), 3.71 (s, 6H).
[0028] Carbon NMR spectrum: (126MHz, DMSO-d 6 )(δ, ppm): 154.31, 152.93, 132.92, 119.93, 113.94, 55.15.
[0029]
Embodiment 2
[0030] Embodiment 2: Preparation of 1,3-bis(4-methylphenyl)urea
[0031] In a 500mL single-necked flask, add 4-methylphenylhydroxamic acid (formula Ⅰ-2, R 1 =4-CH 3 ) 21.16g (140mmol), 150mL dichloromethane, 63.94g (3.0eq, 420mmol) DBU, in SO 2 f 2 In the atmosphere, stir at 25°C for 1 h, filter after the reaction, and rinse the filter cake with acetonitrile until white to obtain 15.64 g of 1,3-bis(4-methylphenyl)urea, with a yield of 93%.
[0032] Proton NMR spectrum: (500MHz, DMSO-d 6 ) (δ, ppm): 8.49 (s, 2H), 7.33 (d, J = 8.4Hz, 4H), 7.08 (d, J = 8.2Hz, 4H), 2.24 (s, 6H).
[0033] Carbon NMR spectrum: (126MHz, DMSO-d 6 )(δ, ppm): 152.62, 137.20, 130.51, 129.14, 118.24, 20.32.
[0034]
Embodiment 3
[0035] Embodiment 3: Preparation of 1,3-bis(3-chlorophenyl)urea
[0036] In a 500mL single-necked flask, add 3-chlorophenylhydroxamic acid (formula Ⅰ-3, R 1 =3-Cl) 24.02g (140mmol), 150mL acetonitrile, 18.10g (3.0eq, 420mmol) Na 2 CO 3 , at SO 2 f 2 Stir at 30° C. for 6 h in the atmosphere, filter after the reaction, and wash the filter cake with acetonitrile until white to obtain 18.11 g of 1,3-bis(3-chlorophenyl)urea with a yield of 92%.
[0037] Proton NMR spectrum: (500MHz, DMSO-d 6 ) (δ, ppm): 9.81 (s, 2H), 7.69 (s, 2H), 7.36–7.24 (m, 4H), 7.02 (dt, J = 6.7, 2.2 Hz, 2H).
[0038] Proton NMR spectrum: (126MHz, DMSO-d 6 )(δ, ppm): 152.46, 141.18, 133.18, 130.40, 121.44, 117.28, 116.36.
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com