Preparation method for 3-(4-methyl-1H-imidazole-1-yl)-5-(trifluoromethyl)aniline
A technology of trifluoromethylbenzoic acid and trifluoromethyl, applied in the field of preparation of 3--5-aniline, can solve the problems of low yield and high cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
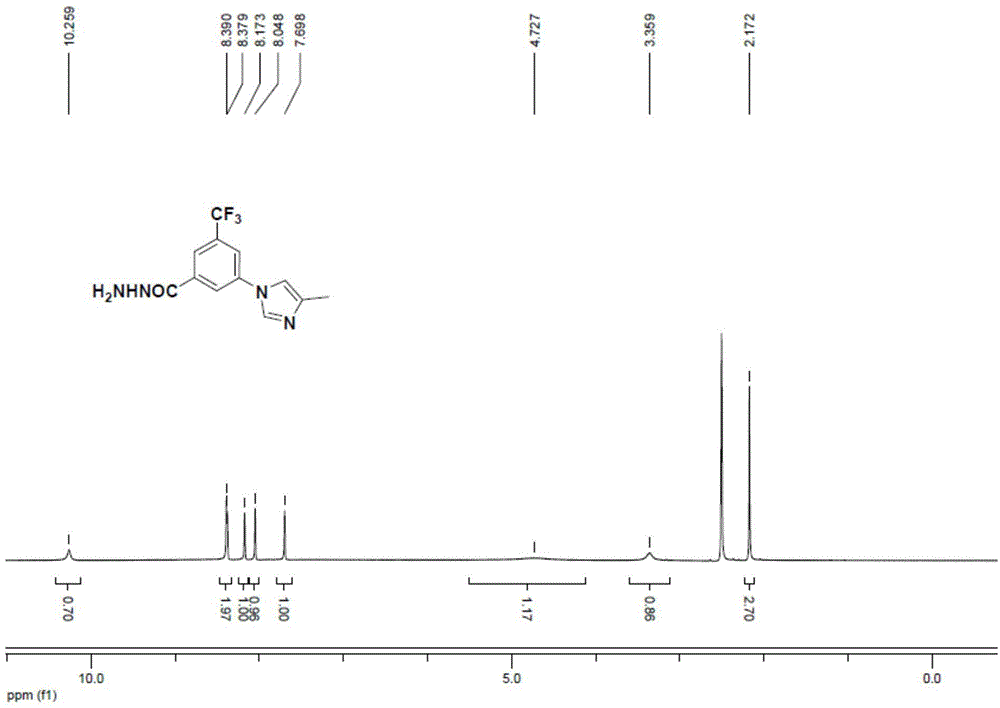
[0046]As described in the background technology section, the existing method for preparing 3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline has the defects of high raw material cost and low yield . In order to solve this problem, the present inventor provides a kind of preparation method of 3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline, which comprises the following steps: S1 , carry out coupling reaction between compound A and 4-methyl-1H-imidazole to generate a coupled product; S2, carry out hydrazinolysis reaction on the coupled product to generate a hydrazinolysis product; and S3, subject the hydrazinolysis product to diazonium in turn Chemical reaction, Curtius rearrangement reaction, generate 3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline; Wherein, compound A is selected from 3-halo-5-trifluoro Toluic acid, salt derivatives of 3-halo-5-trifluoromethylbenzoic acid or ester derivatives of 3-halo-5-trifluoromethylbenzoic acid.
[0047] The abo...
Embodiment 1
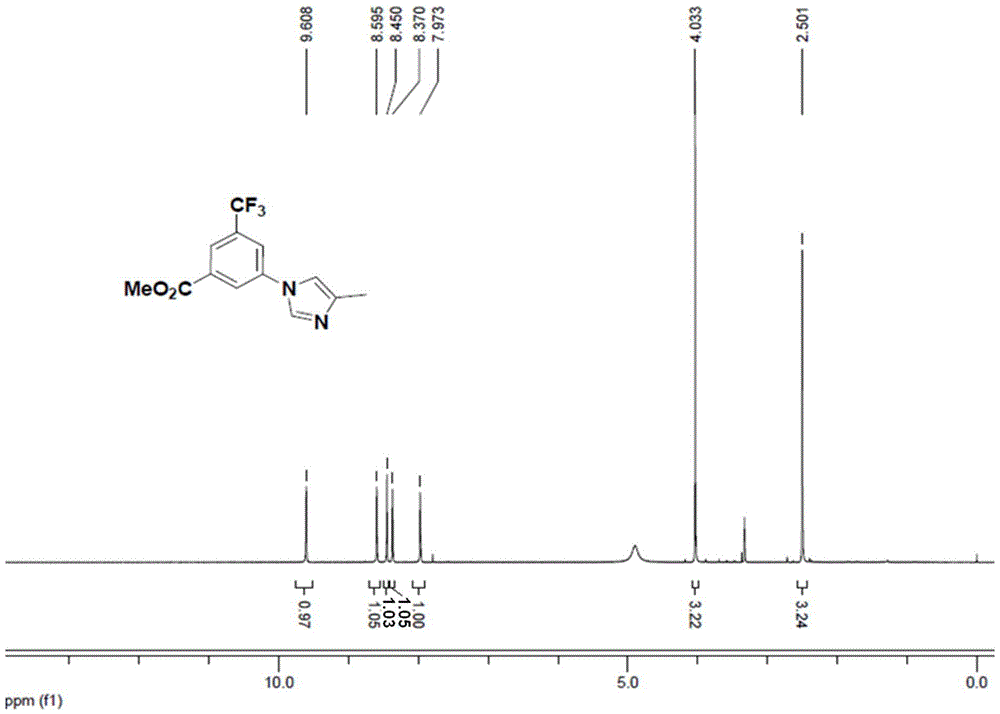
[0078] Coupling reaction: Add methyl 3-bromo-5-trifluoromethylbenzoate (compound 2, 200g, 0.707mol), 4-methyl-1H-imidazole (116g, 1.41mol), trans-N , N'-dimethylcyclohexanediamine (20g, 0.14mol), DMF (8vol / g, relative to compound 2), anhydrous potassium phosphate (300g, 1.41mol) and 4A molecular sieve (0.1g / g), A mixed system is formed; the mixed system is replaced with nitrogen to oxygen content ≤ 500ppm, and finally cuprous iodide (26.9g, 0.14mol) is added to form a system to be reacted; Track the reaction until compound 2 disappears (44 hours) to obtain the product system; then the system is cooled to room temperature, quenched in 2M hydrochloric acid to obtain the solution to be purified; ethyl acetate is added to the solution to be purified, and the organic phase is obtained by extraction and separation , the aqueous phase was extracted twice with ethyl acetate, and the organic phases were combined; the organic phase was concentrated to remove the ethyl acetate solvent to...
Embodiment 2
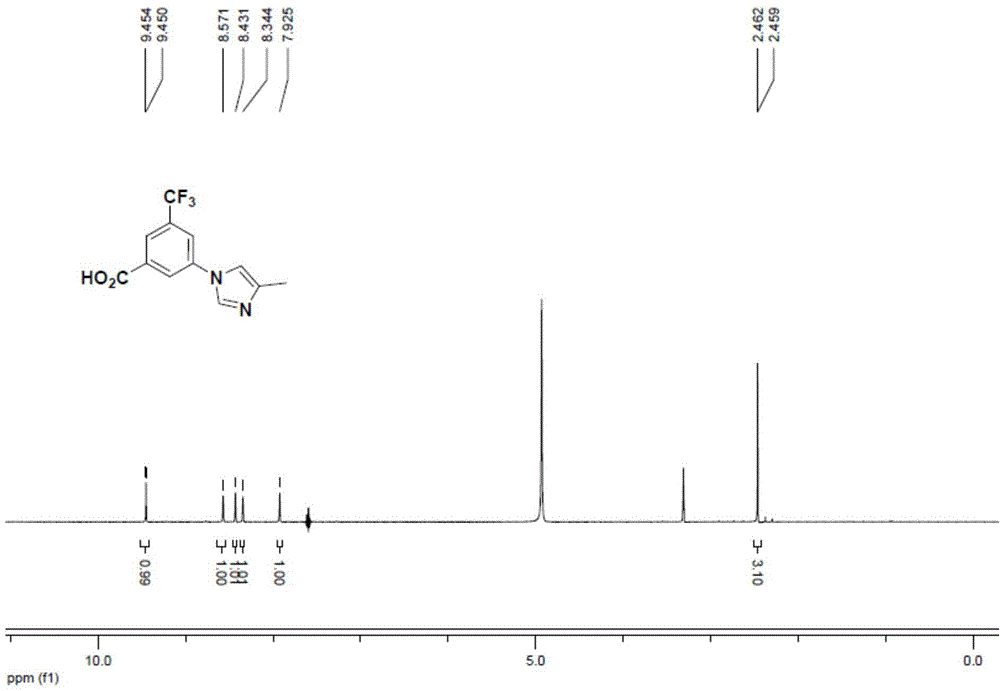
[0083] Coupling reaction: Add 3-bromo-5-trifluoromethylbenzoate sodium (compound 2, 29g, 0.1mol), 4-methyl-1H-imidazole (16.4g, 0.2mol), trans-N ,N'-Dimethylcyclohexanediamine (2.8g, 0.02mol), DMF (8vol. / g), anhydrous potassium phosphate (42g, 0.2mol) and 4A molecular sieve (0.1g / g) to form a mixed system ; The mixed system is replaced with nitrogen to oxygen content ≤ 500ppm, and finally copper iodide (3.8g, 0.02mol) is added to form a system to be reacted; the system to be reacted; the temperature is raised to 115 ° C, and the reaction is stirred for 60 hours, TLC tracking showed that compound 2 disappeared to obtain the product system; the product system was cooled to 30-35° C., and bromoethane (30 g, 0.275 mol) was added dropwise thereto, and the coupling carboxylic acid generated in situ was completely ethylated by TLC tracking. The system was lowered to room temperature, quenched with 2M hydrochloric acid, added ethyl acetate, extracted and separated to obtain the organi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com