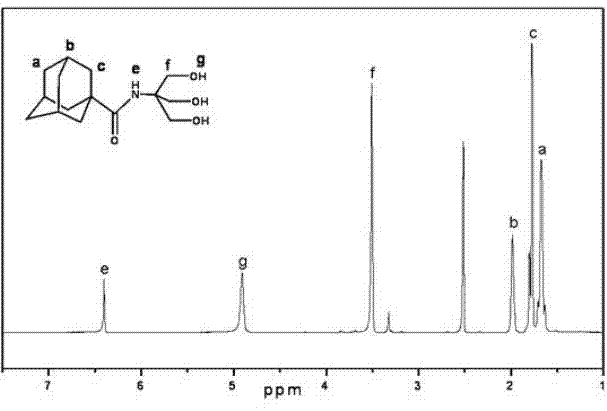
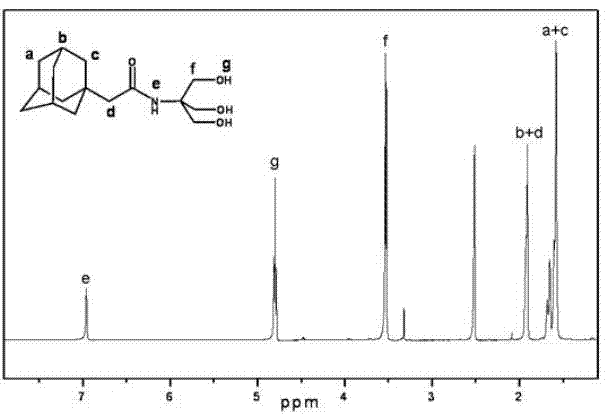
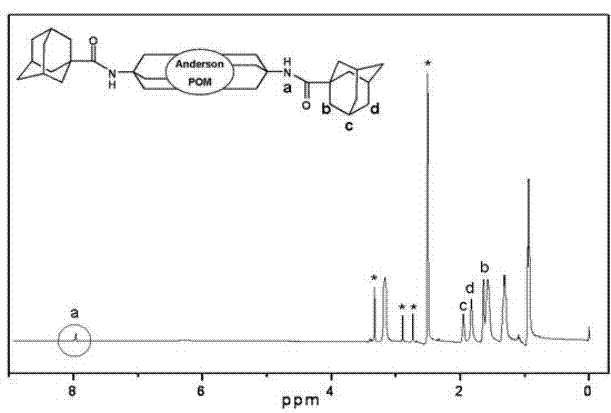
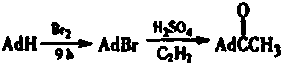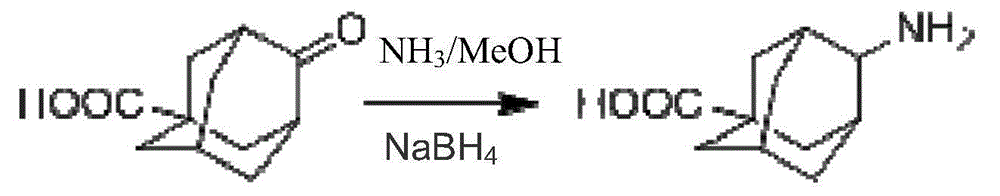Patents
Literature
47 results about "Adamantanecarboxylic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Molybdenum-containing polyoxometalate and adamantane hybrid compound and preparation method thereof
InactiveCN102304152AEasy to prepareReaction is easy to controlOrganic chemistrySynthesis methodsHybrid compound
The invention discloses a molybdenum-containing polyoxometalate and adamantane hybrid compound, which is prepared by connecting molybdenum-containing polyoxometalate and adamantane through a covalent bond. The preparation method comprises the following synthesis steps of: 1) performing amidation reaction on adamantane carboxylic acid serving as an initial raw material and trishydroxymethyl aminomethane to obtain N-trishydroxymethyl-adamantane carboxamide; and 2) reacting with the molybdenum-containing polyoxometalate to obtain the hybrid compound. By the invention, the molybdenum-containing polyoxometalate and the adamantine are connected through the covalent bond for the first time; and the synthesis method is simple and feasible, reaction conditions are easy to realize, purification is simple, and the yield is high. The hybrid molecule has potential application value in the aspects of medicines, lubricants, surfactants, catalysts and the like.
Owner:NANKAI UNIV
Synthesis of magnesium adamantane salts and magnesium oxide nanocomposites, and systems and methods including the salts or the nanocomposites
ActiveUS20170267620A1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMagnesium saltCarboxylic salt
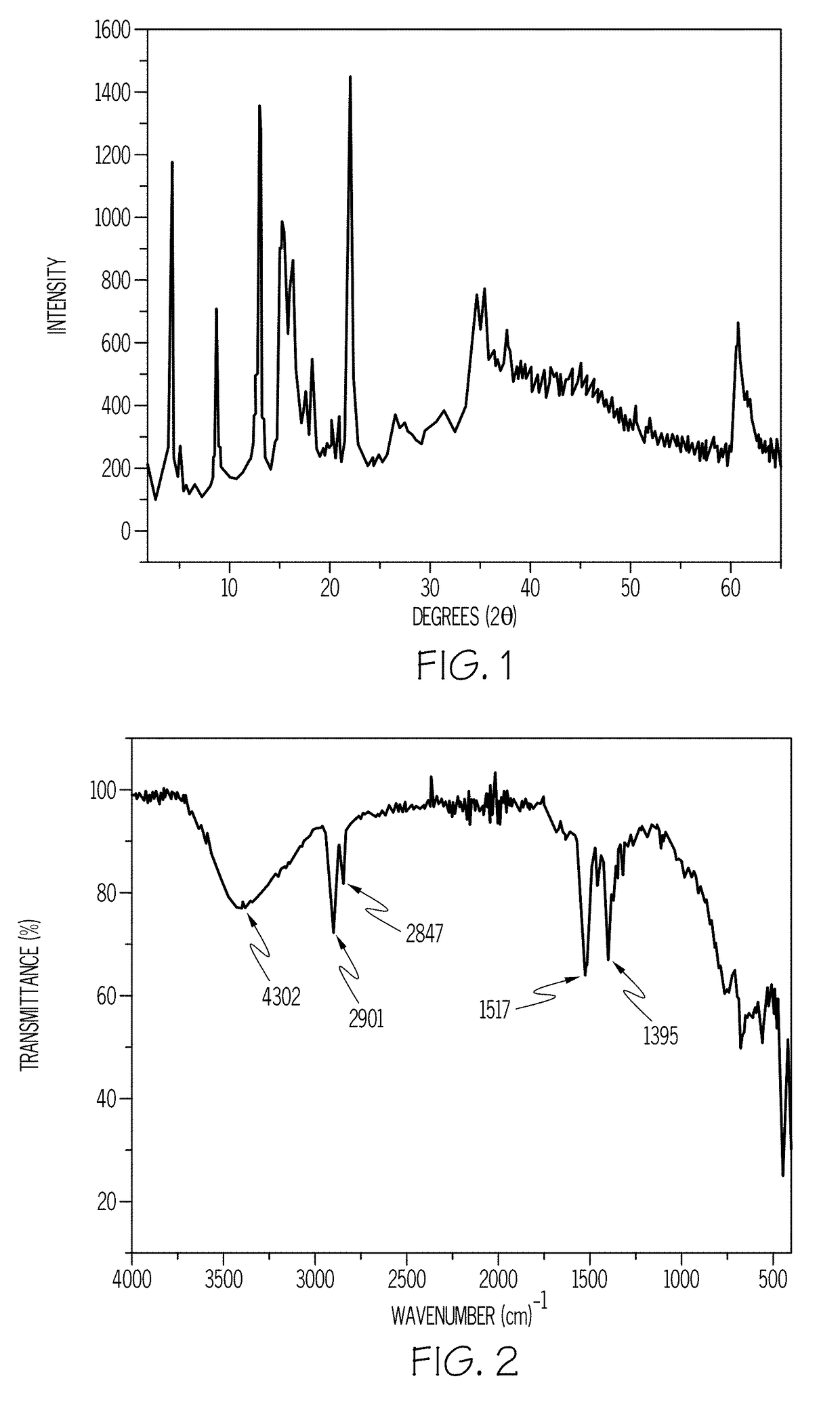
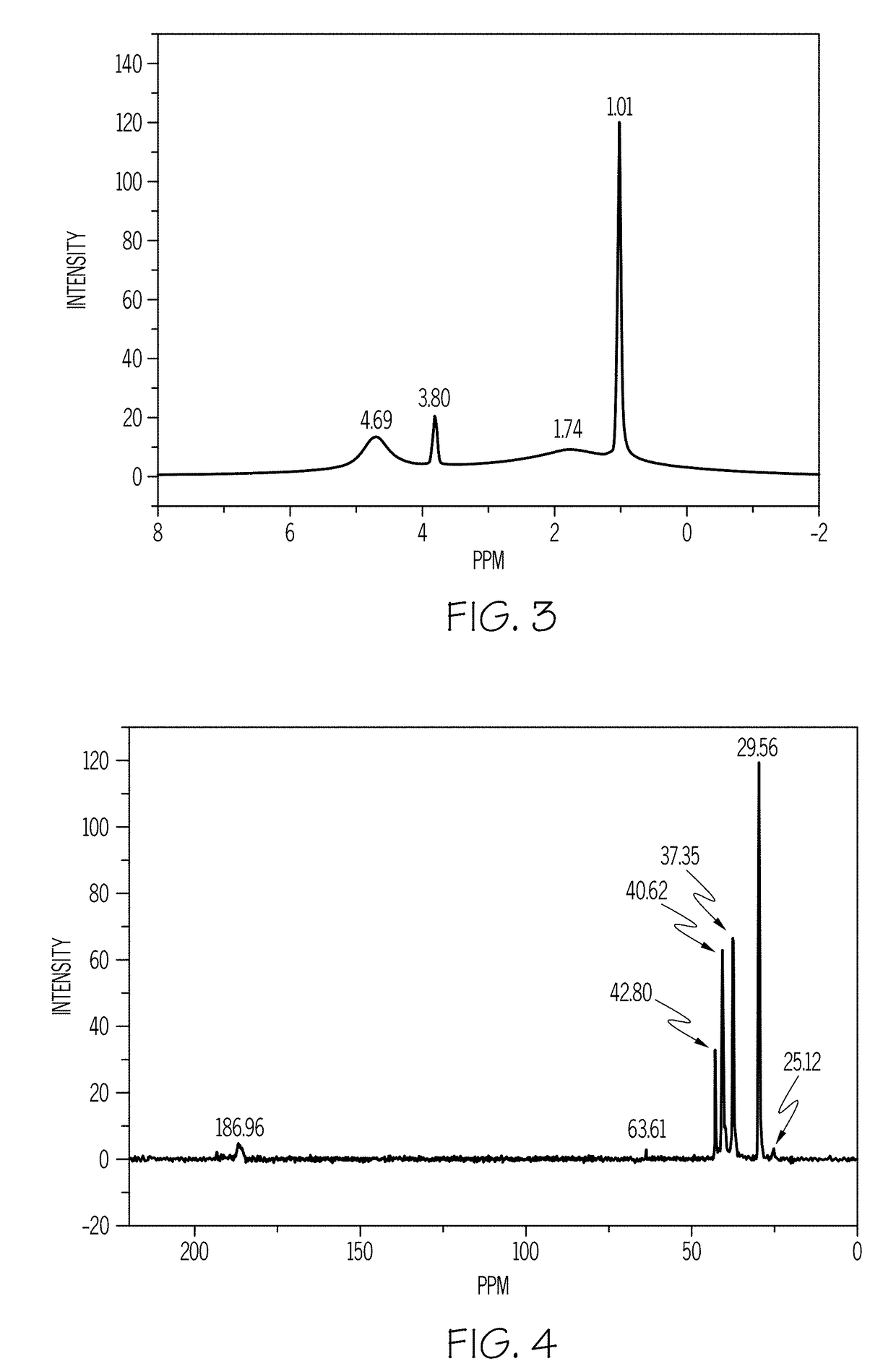
A method for preparing a magnesium adamantane carboxylate salt is provided. The method includes mixing a magnesium salt and a diamondoid compound having at least one carboxylic acid moiety to form a reactant mixture and hydrothermally treating the reactant mixture at a reaction temperature for a reaction time to form the magnesium adamantane carboxylate salt.
Owner:SAUDI ARABIAN OIL CO +1
High temperature layered mixed-metal oxide materials with enhanced stability
ActiveUS20170266642A1Improve thermal stabilityImprove abilitiesAluminium compoundsGas treatmentReaction temperatureLayered double hydroxides
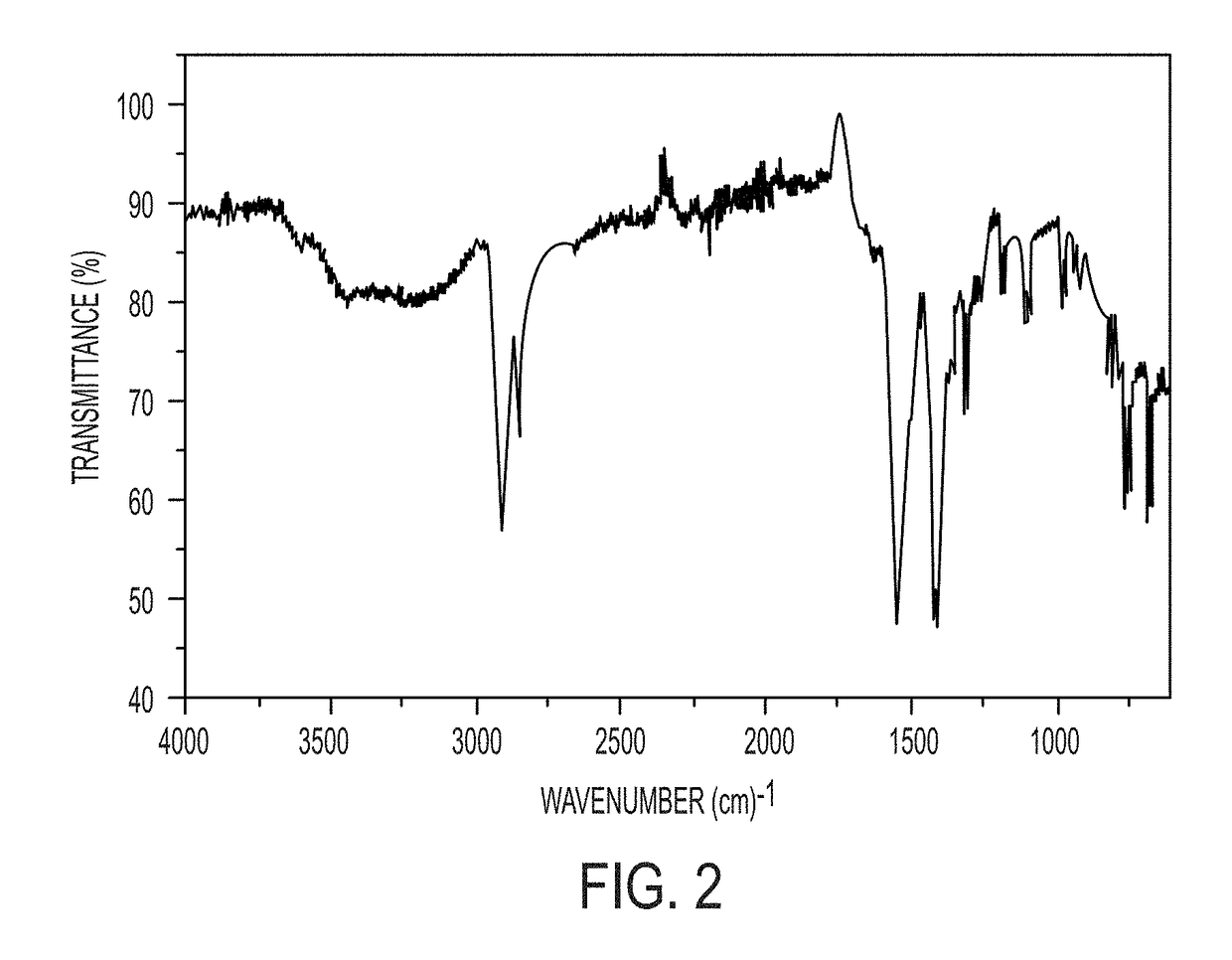
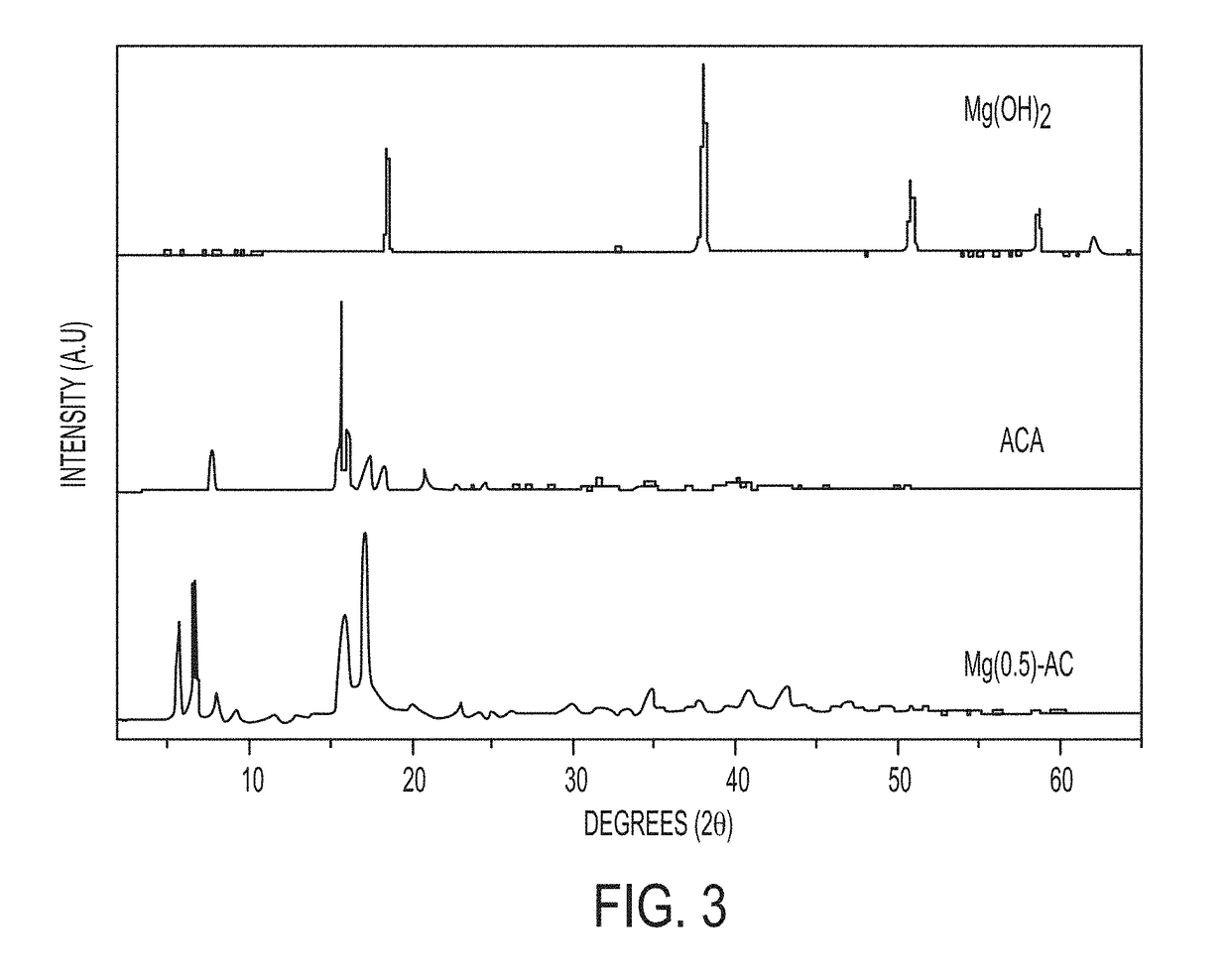
Embodiments of the present disclosure are directed towards methods for preparing mixed-metal oxide particles by heating adamantane-intercalated layered double-hydroxide (LDH) particles at a reaction temperature of from 400° C. to 800° C. to form mixed-metal oxide particles. The adamantane-intercalated LDH particles have a general formula [M1-xAlx(OH)2](A)x.mH2O, where x is from 0.14 to 0.33, m is from 0.33 to 0.50, M is chosen from Mg, Ca, Co, Ni, Cu, or Zn, and A is adamantane carboxylate, and an aspect ratio greater than 100. The aspect ratio is defined by the width of an adamantane-intercalated LDH particle divided by the thickness of the adamantane-intercalated LDH particle. The mixed-metal oxide particles comprise a mixed-metal oxide phase containing M, Al or Fe, and carbon.
Owner:SAUDI ARABIAN OIL CO +1
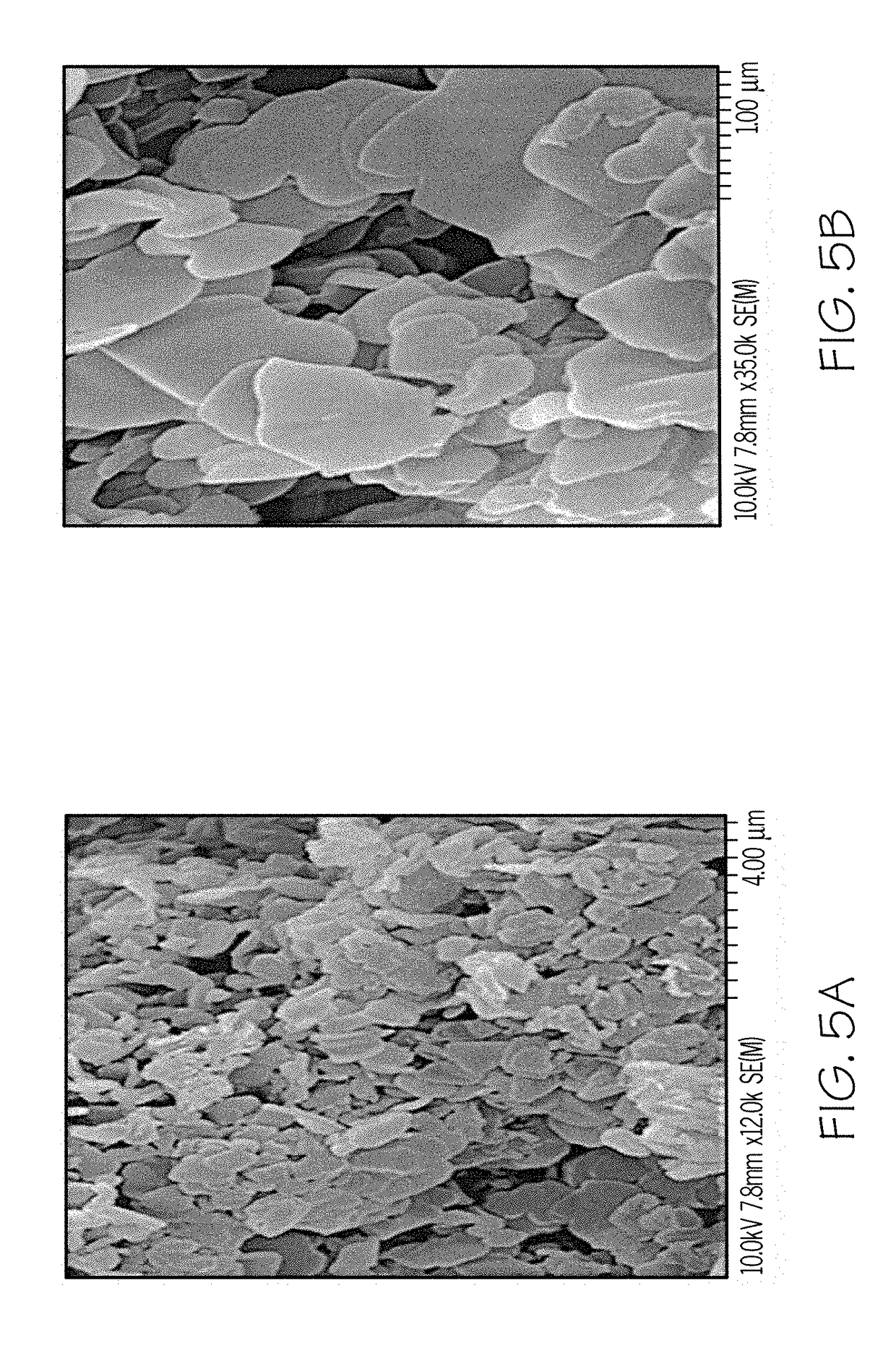
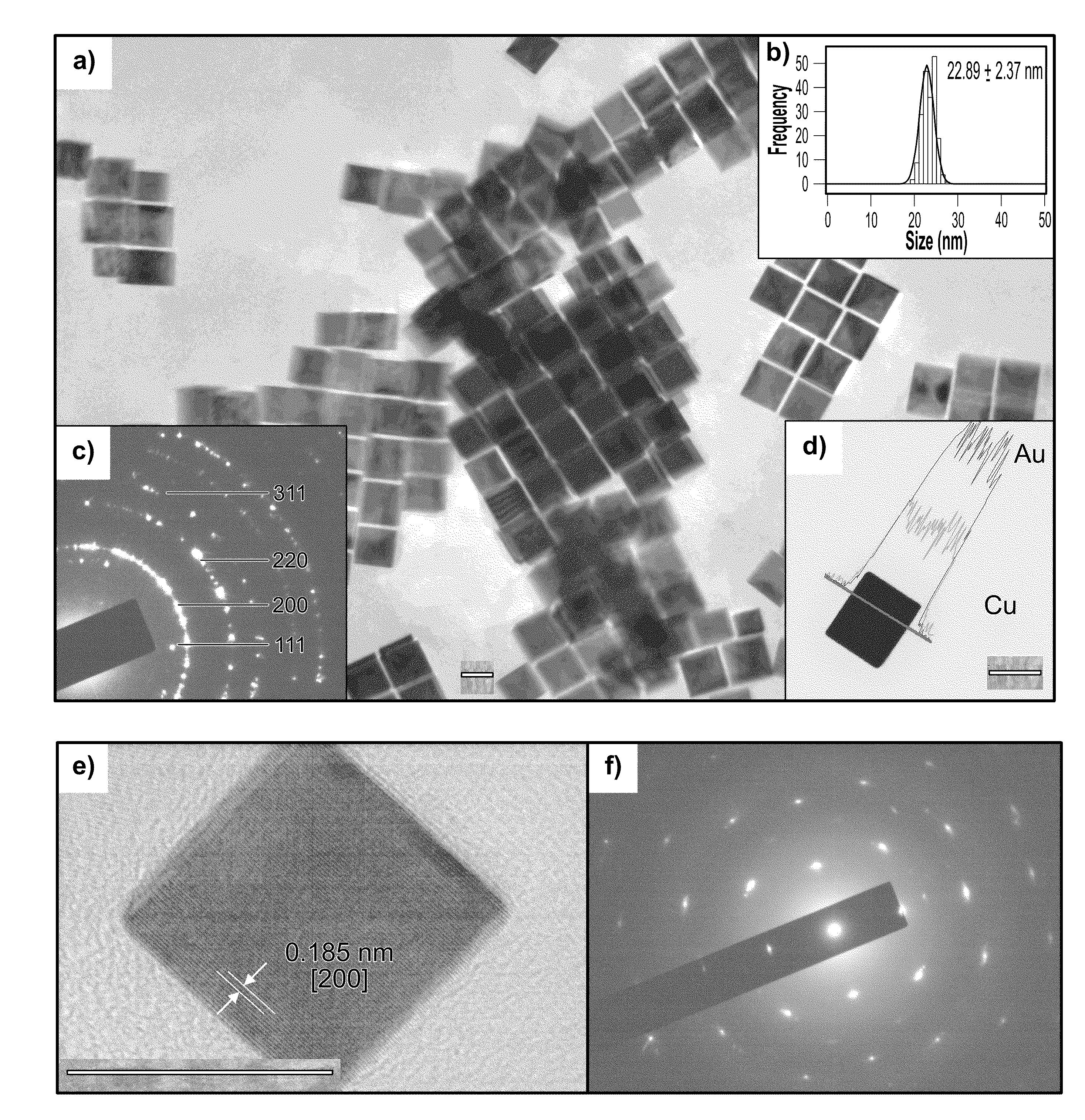
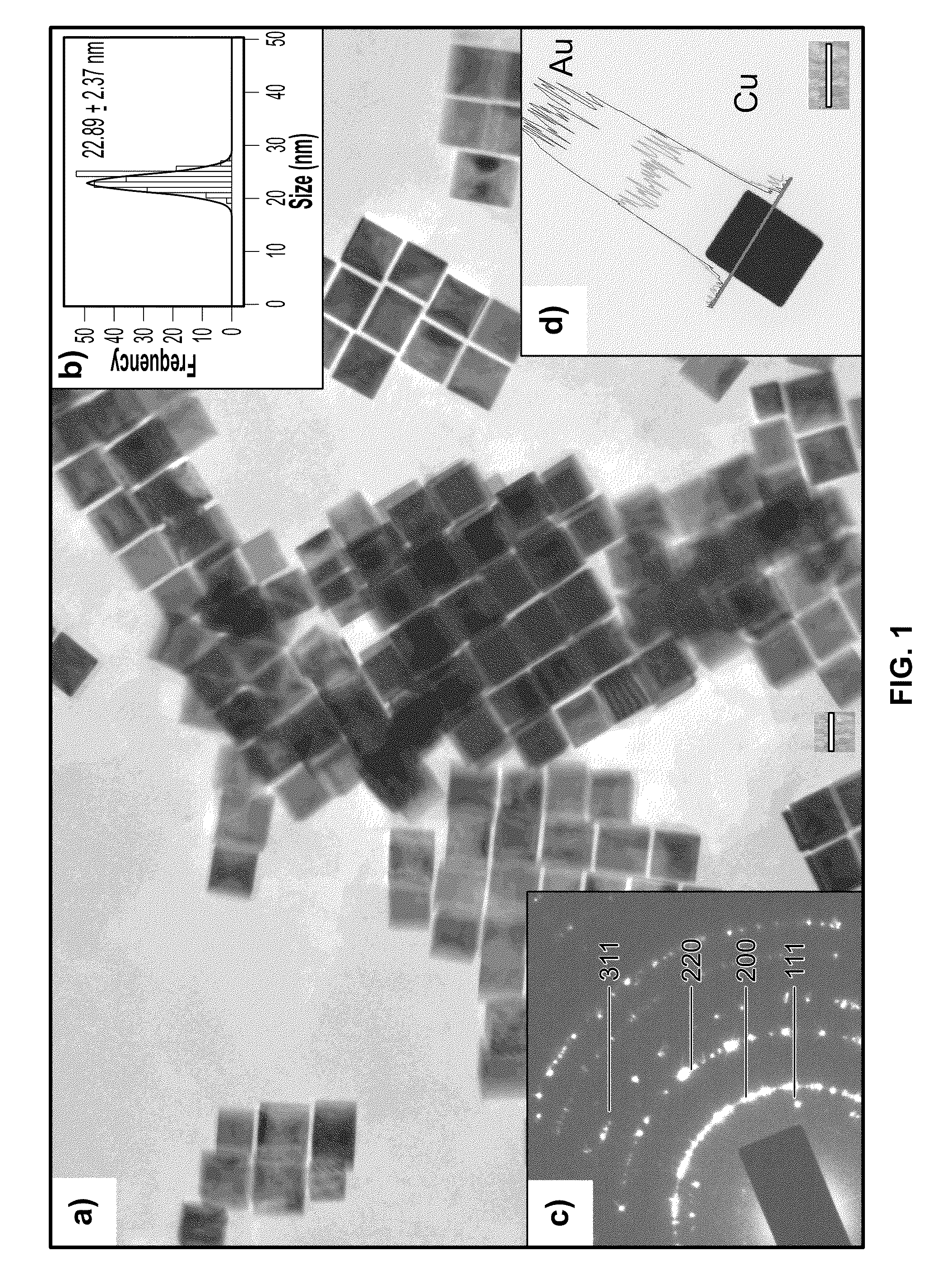
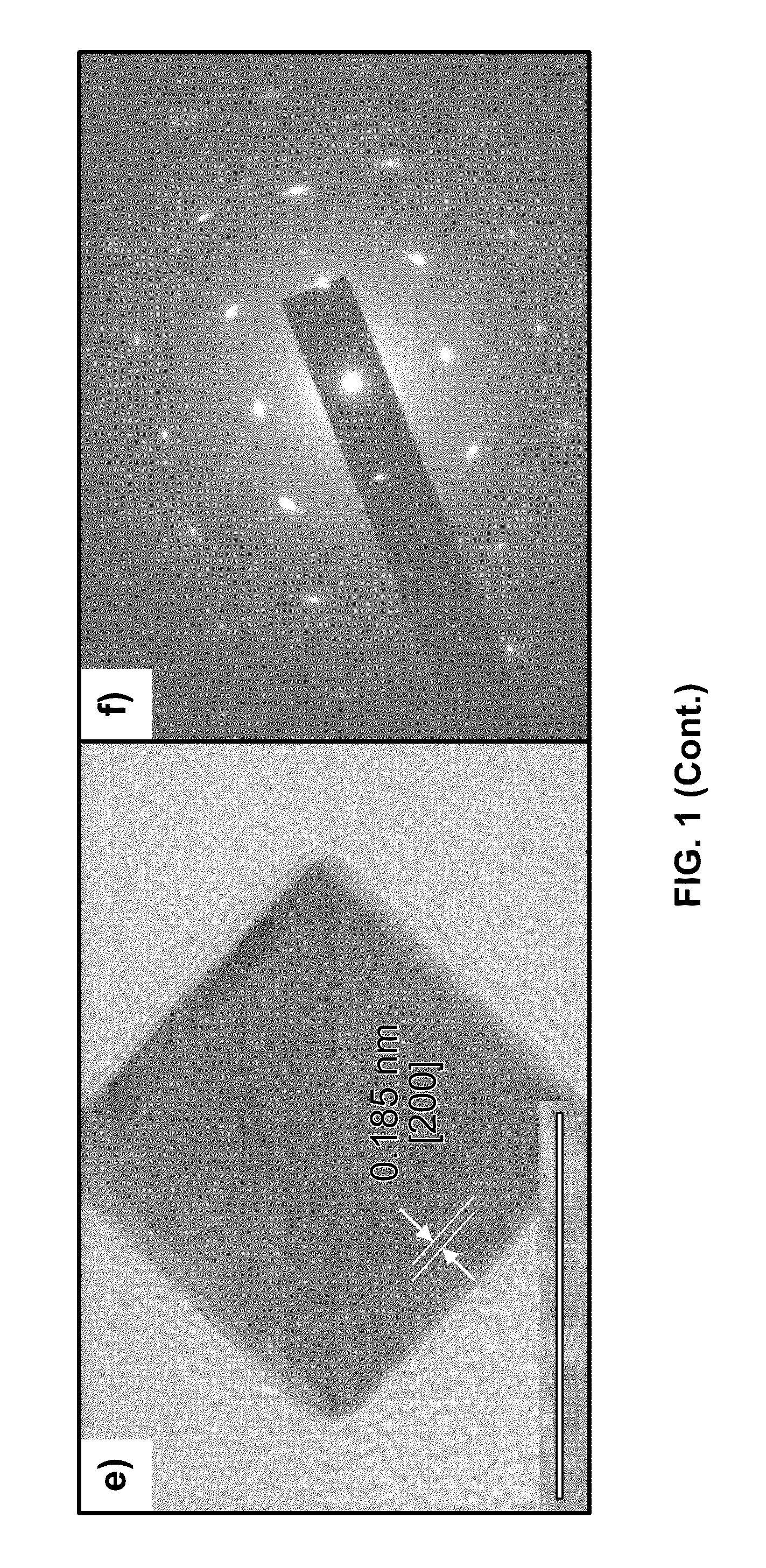
One-step synthesis of monodisperse au-cu nanocubes
ActiveUS20110283834A1Material nanotechnologyTransportation and packagingDiphenyl etherCelsius Degree
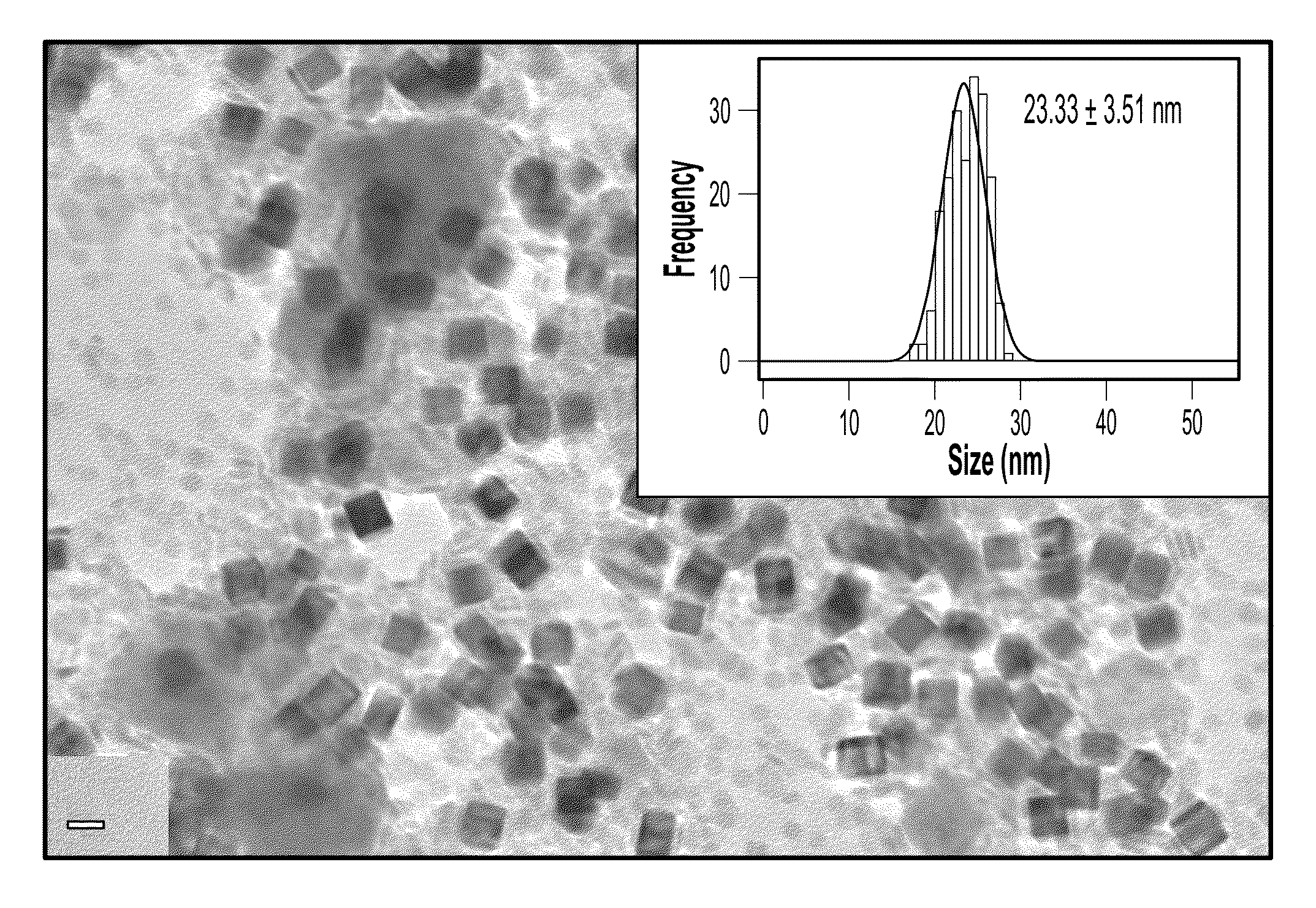
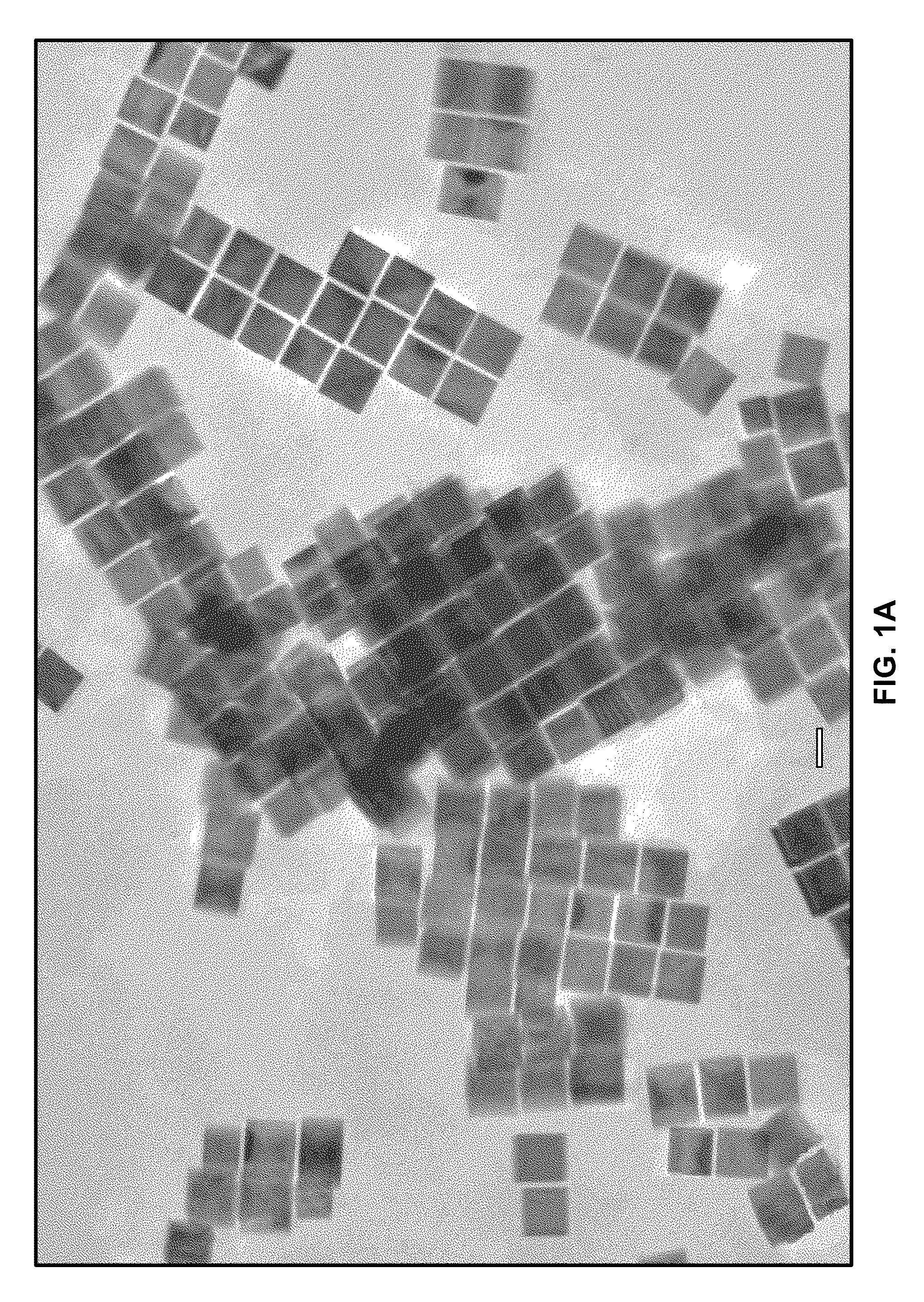
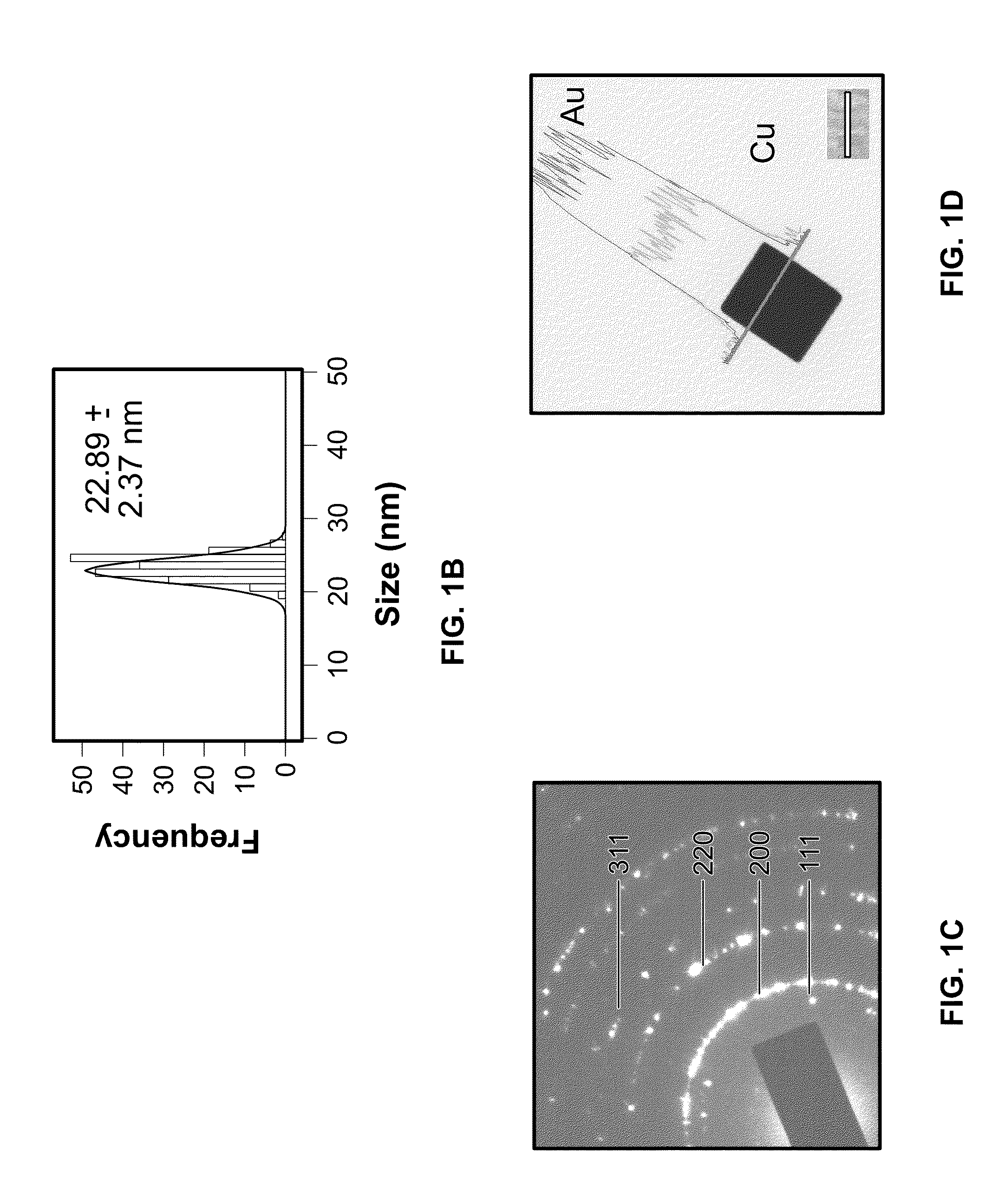
A one-step process for synthesizing gold-copper bimetallic nanocubes. The process comprises the step of simultaneously reducing a copper II salt and a gold halide by 1,2-hexadecanediol in diphenyl ether, and 1-dodecanethiol as well as surfactants 1-adamantanecarboxylic acid and 1-hexadecylamine. The copper II salt may be copper (II) acetylacetonate, copper chloride, copper sulfate, or copper phosphate. The gold halide may be chloroauric acid, gold chloride, gold bromide, or tetrabromoauric acid. The reduction may occur at a temperature between about 160 and 180 degrees Celsius. The copper II salt may be copper (II) acetylacetonate and the gold halide may be chloroauric acid.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF COMMERCE THE NAT INST OF STANDARDS & TEHCNOLOGY
Method for separation and purification of 2-adamantanecarboxylic acid and 1-adamantanecarboxylic acid
InactiveCN102249900ATake advantage ofAvoid pollutionOrganic compound preparationCarboxylic compound preparationSocial benefitsReaction temperature
The invention relates to a method for separation and purification of an adamantanecarboxylic acid mixture, especially a method for separation and purification of 2-adamantanecarboxylic acid and 1-adamantanecarboxylic acid. Comprising the steps of esterification and separation, the method is characterized in that: in the esterification reaction, 98% sulfuric acid is employed as a catalyst, with the ratio of the catalyst to raw materials in a range of from 0.5-3:10, the reaction time is 15-140min, and the reaction temperature ranges from 55-70DEG C; then water and NaOH are added to make the pH value of the solution above 10, so that a water and an ester layer is separated. In the invention, the separation of 1-adamantanecarboxylic acid and 2-adamantanecarboxylic acid is realized through an esterification reaction, i.e. through the esterification differences between 2-adamantanecarboxylic acid and 1-adamantanecarboxylic acid. Therefore, not only is the method of the invention simple and practicable, but also the product generated after separation and purification has satisfying purity. Especially, residues from adamantanecarboxylic acid production can be fully utilized, so that the discharge of "three wastes" can be reduced and environmental pollution is prevented. With remarkable economic benefit and social benefit, the method of the invention is economical and environmental friendly.
Owner:ZHEJIANG NORMAL UNIVERSITY
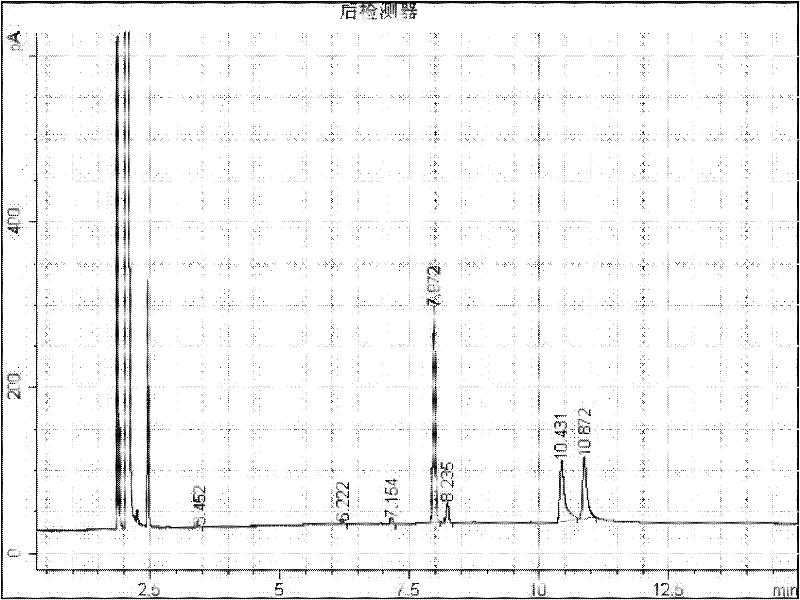
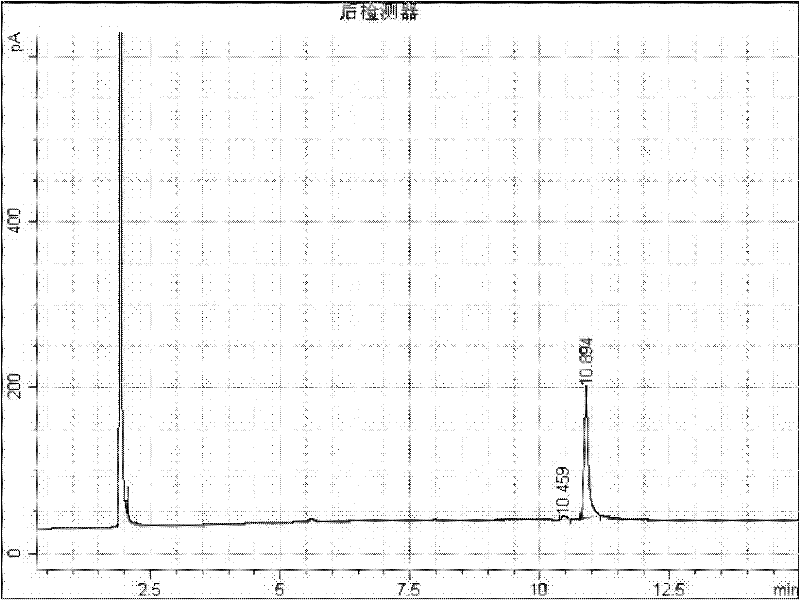
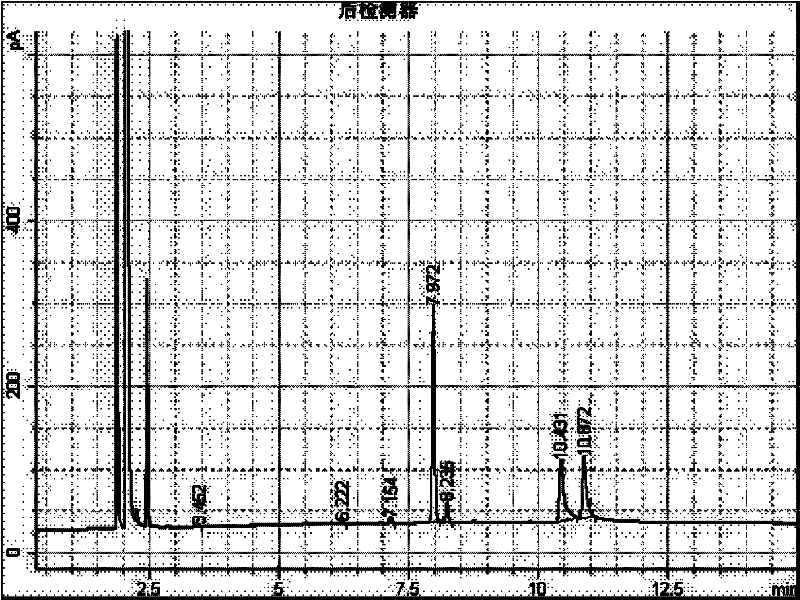
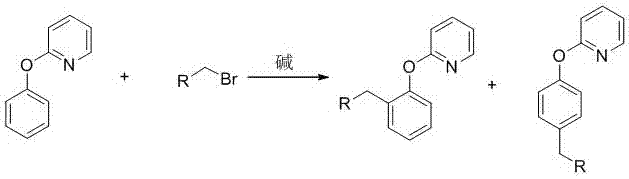
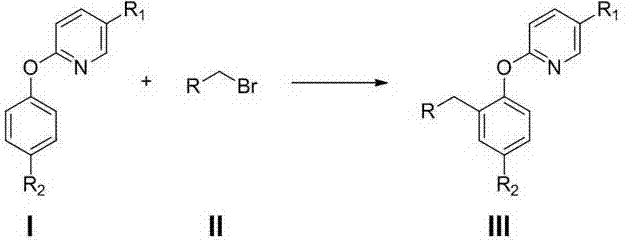
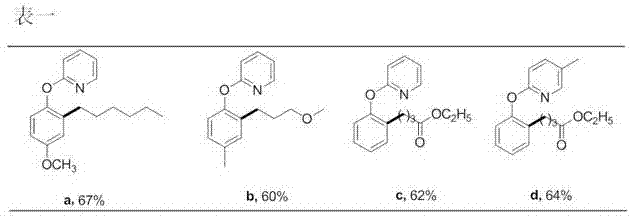
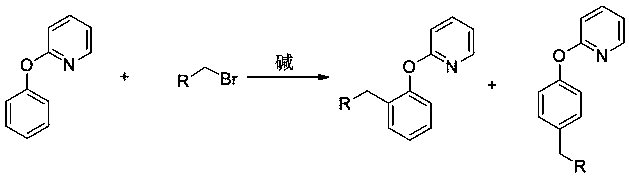
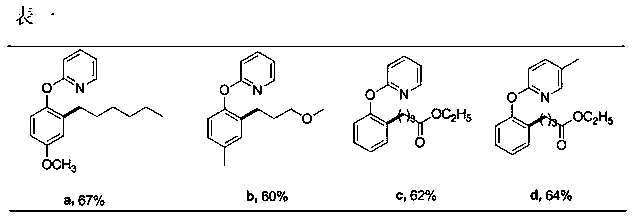
Preparation method of 2-(2-alkylphenoxy)pyridine derivative
InactiveCN107043350AHigh puritySynthetic raw materials are readily availableOrganic chemistryCyclohexanecarboxylic acidGlycol synthesis
A preparation method of a 2-(2-alkylphenoxy)pyridine derivative adopts a reaction of a 2-phenoxypyridine derivative and a primary bromoalkane compound. The method concretely comprises the following steps: directly adding the 2-phenoxypyridine derivative, the primary bromoalkane compound, a catalyst, an additive, an alkali and a solvent into a reaction device, stirring and heating the above added substances until the temperature is 90-130 DEG C, carrying out a reaction for 18-36 h, and separating the obtained product to obtain the 2-(2-alkylphenoxy)pyridine derivative, wherein the catalyst is dichloro(p-cymene)ruthenium dimer; the alkali is potassium carbonate or lithium carbonate; the additive is 1-adamantanecarboxylic acid or naphthenic acid; and the solvent is benzene or DMF or ethylene glycol dimethyl ether. The method has the advantages of easiness in obtaining of synthesis raw materials, simple process, and very good specificity of the reaction.
Owner:ANYANG NORMAL UNIV
Method for preparing water-soluble rare-earth nanometer particles by super molecular self assembly
ActiveCN101851001ARaw materials are easy to getLow costNanostructure manufactureRare earth metal compoundsSolubilityRare earth
The invention belongs to the technical field of nanometer and super molecules, in particular to a method for preparing water-soluble rare-earth nanometer particles by super molecular self assembly. The method mainly comprises the following steps: firstly, synthesizing oil-soluble rare-earth nanometer particles modified by adamantine carboxylic acid; then, adding beta-cyclodextrin, and converting the beta-cyclodextrin into hydrophilic rare-earth nanometer particles through the strong super molecular effect with the adamantine; and carrying out centrifugal separation for washing away excrescent beta-cyclodextrin. The method has the advantages of easy acquisition of raw materials, low cost, simple process and fast and convenient reaction, the obtained rare-earth nanometer particles have good water solubility, and can be easily connected with other molecules through the cyclodextrin, and the functionalization of the rare-earth nanometer particles is realized.
Owner:中山泰辉生物科技有限公司
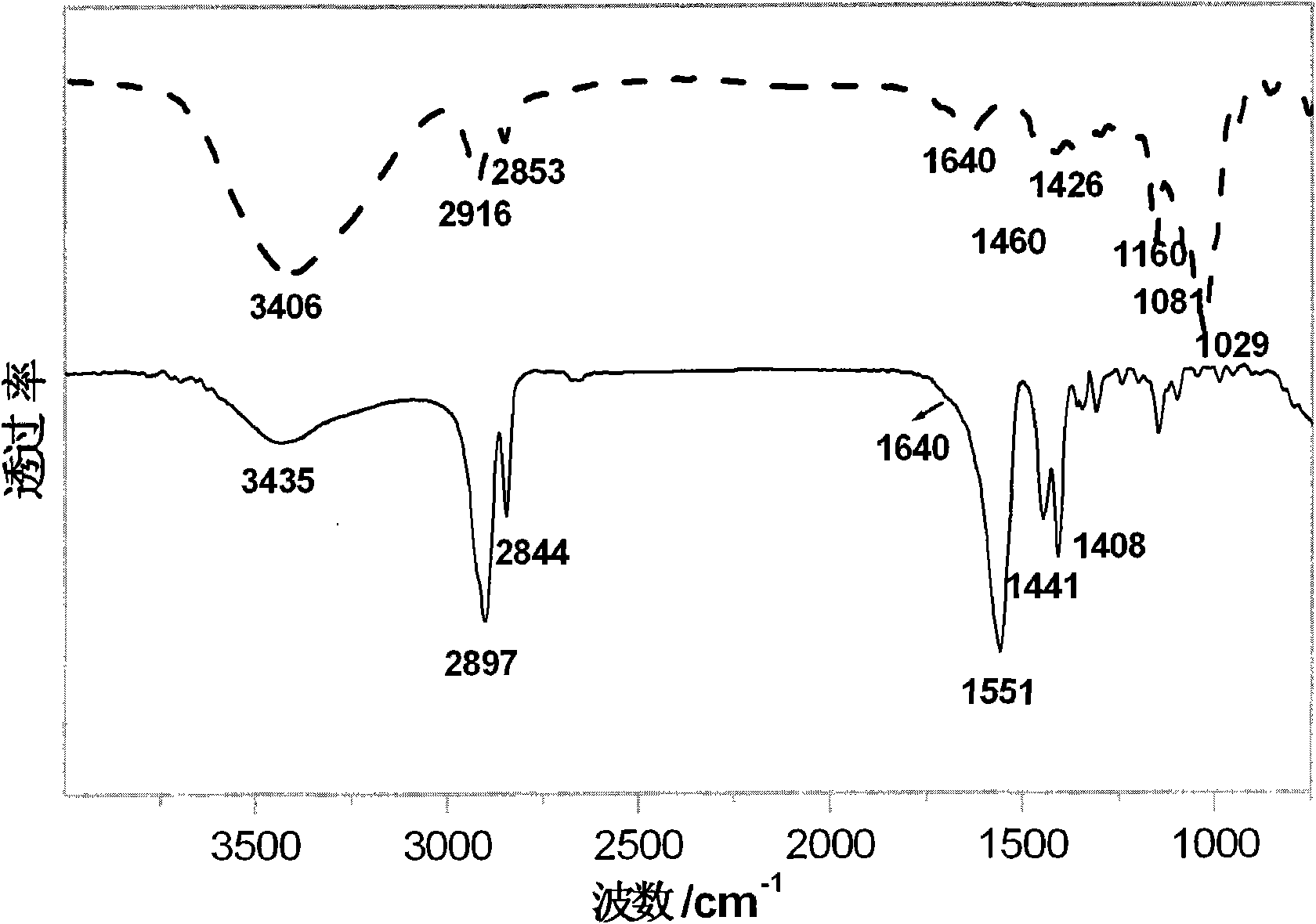
Method for synthesizing 3-amino-1-adamantanol
InactiveCN101747212AEasy to operateHigh yieldOrganic compound preparationAmino-hyroxy compound preparationBromateCurtius rearrangement
The invention discloses a method for synthesizing 3-amino-1-adamantanol. The structural formula of the 3-amino-1-adamantanol is as follows. Using adamantanecarboxylic acid as starting material, the method synthesizes the 3-amino-1-adamantanol according to the following reaction steps: bromate 3-amino-1-adamantanol is synthesized by way of bromination, modified curtius rearrangement and hydrolyzation, and finally, bromate is removed by a alkaline liquor treatment method, so that the 3-amino-1-adamantanol is obtained. The invention has the advantages of simple and easy operation, available materials, less reaction steps and high product yield.
Owner:GUANGDONG UNIV OF TECH
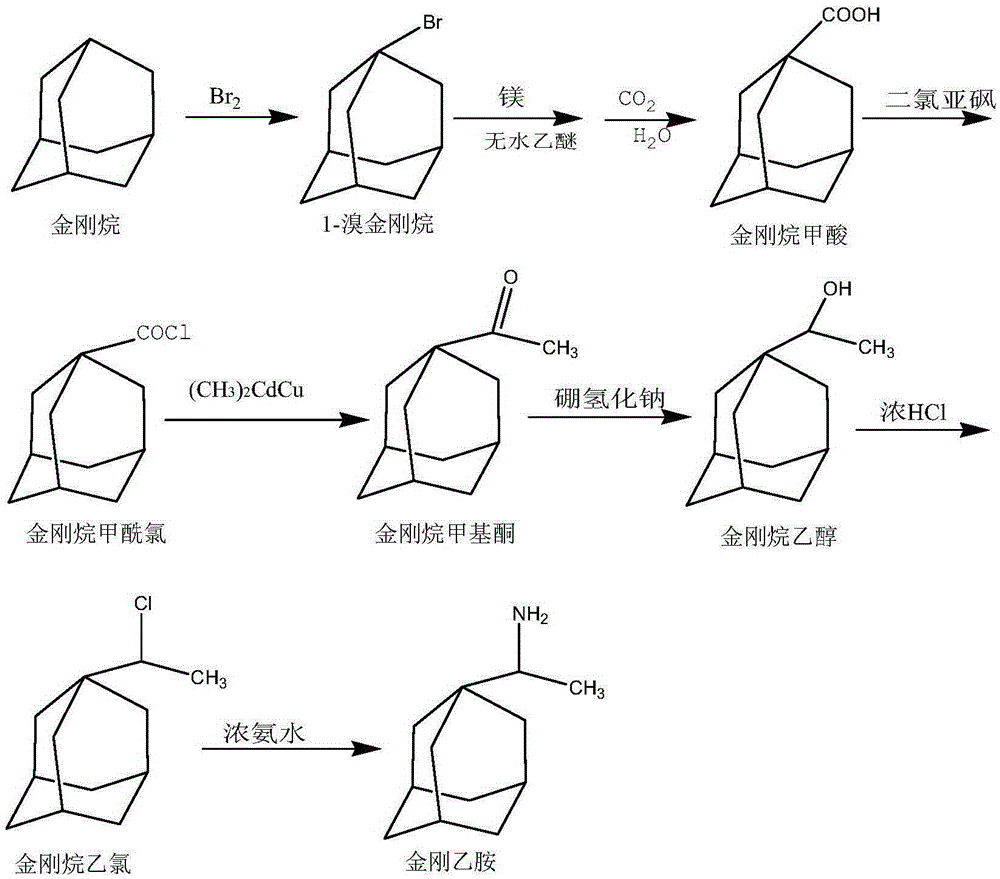
A kind of synthetic method of rimantadine
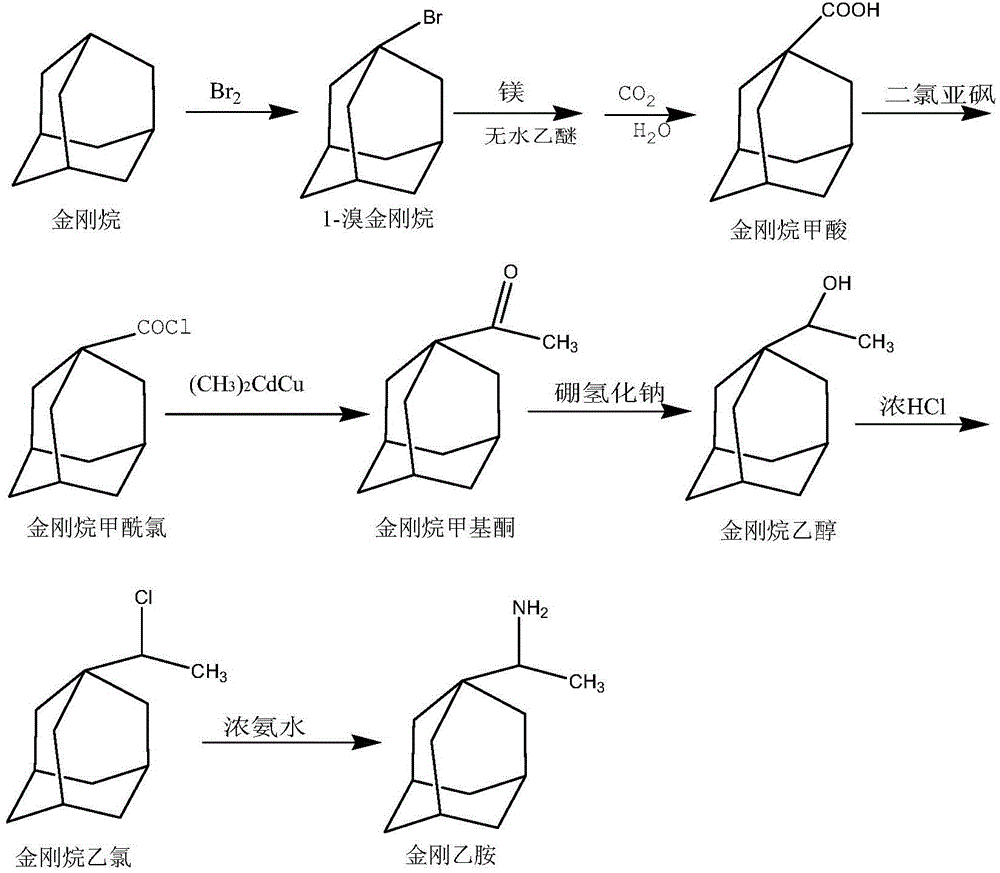
InactiveCN104610067BMild conditionsEasy to follow upAmino preparation by functional substitutionMethyl palmoxirateHydrochloric acid
The invention discloses a synthetic method for rimantadine. The synthetic method is characterized by comprising the following steps: firstly, obtaining 1-bromoadamantane by reacting adamantine with liquid bromine; then, acidifying to obtain adamantanecarboxylic acid after reacting 1-bromoadamantane with magnesium and anhydrous ether; obtaining adamantine carbonyl chloride by performing reflux reaction on tehadamantanecarboxylic acid with thionyl chloride; obtaining adamantane methyl ketone by reacting the adamantine carbonyl chloride with (CH3)2CdCu; and finally, obtaining the rimantadine by hydriding and reacting adamantane methyl ketone with hydrochloric acid and ammonia water in the presence of sodium borohydride. The synthetic method disclosed by the invention is gentle in condition, simple in follow-up processing, high in yield, cheap in raw material and low in synthesis cost.
Owner:ANHUI UNIV OF SCI & TECH
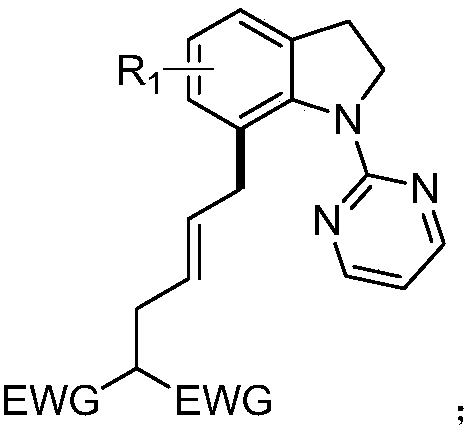
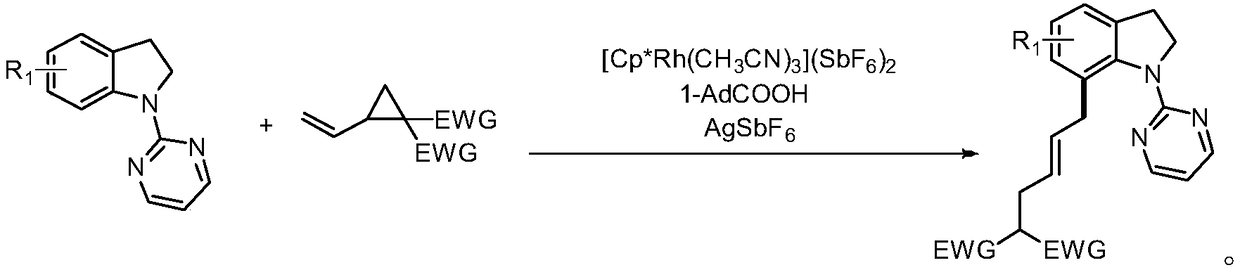
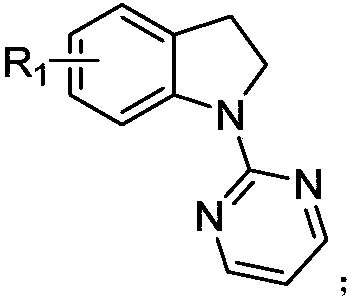
7-alkyl-N-pyrimidylindoline compound and synthesis method thereof
ActiveCN109180648ARich types of functionalization reactionsMultiple Feasibility PathwaysOrganic chemistryChromatographic separationCarbon–carbon bond
The invention discloses a 7-alkyl-N-pyrimidylindoline compound and a synthesis method thereof. The 7-alkyl-N-pyrimidylindoline compound has a general structural formula shown in the description. The synthesis method comprises: adding an N-pyrimidylindoline compound and a vinyl cyclopropane compound into a reaction tube in an argon environment, adding 1-adamantanecarboxylic acid, [Cp*Rh(CH3CN)3](SbF6)2 and silver hexafluoroantimonate into the reaction tube, then adding methanol into the reaction tube, carrying out a reaction process at 80-120 DEG C for 12h, and then carrying out leaching, thinlayer chromatographic separation and drying to obtain a desired product. The metal ruthenium (III) is used as a catalyst and methanol is used as a reaction solvent so that the carbon-carbon bond cleavage of vinylcyclopropane and activation of a 7-site carbon-hydrogen bond of an indoline compound are realized. The long alkyl chain with double bonds realizes a further functionalization reaction. Thesynthesis method is simple and efficient and has good selectivity. The 7-alkyl-N-pyrimidylindoline compound is easy to purify.
Owner:ZHENGZHOU UNIV
Synthetic method for rimantadine
InactiveCN104610067AMild conditionsEasy to follow upAmino preparation by functional substitutionMethyl palmoxirateHydrochloric acid
The invention discloses a synthetic method for rimantadine. The synthetic method is characterized by comprising the following steps: firstly, obtaining 1-bromoadamantane by reacting adamantine with liquid bromine; then, acidifying to obtain adamantanecarboxylic acid after reacting 1-bromoadamantane with magnesium and anhydrous ether; obtaining adamantine carbonyl chloride by performing reflux reaction on tehadamantanecarboxylic acid with thionyl chloride; obtaining adamantane methyl ketone by reacting the adamantine carbonyl chloride with (CH3)2CdCu; and finally, obtaining the rimantadine by hydriding and reacting adamantane methyl ketone with hydrochloric acid and ammonia water in the presence of sodium borohydride. The synthetic method disclosed by the invention is gentle in condition, simple in follow-up processing, high in yield, cheap in raw material and low in synthesis cost.
Owner:ANHUI UNIV OF SCI & TECH
High temperature layered mixed-metal oxide materials with enhanced stability
ActiveUS10252245B2Improve thermal stabilityImprove abilitiesGas treatmentAluminium compoundsReaction temperatureLayered double hydroxides
Embodiments of the present disclosure are directed towards methods for preparing mixed-metal oxide particles by heating adamantane-intercalated layered double-hydroxide (LDH) particles at a reaction temperature of from 400° C. to 800° C. to form mixed-metal oxide particles. The adamantane-intercalated LDH particles have a general formula [M1-xAlx(OH)2](A)x.mH2O, where x is from 0.14 to 0.33, m is from 0.33 to 0.50, M is chosen from Mg, Ca, Co, Ni, Cu, or Zn, and A is adamantane carboxylate, and an aspect ratio greater than 100. The aspect ratio is defined by the width of an adamantane-intercalated LDH particle divided by the thickness of the adamantane-intercalated LDH particle. The mixed-metal oxide particles comprise a mixed-metal oxide phase containing M, Al or Fe, and carbon.
Owner:SAUDI ARABIAN OIL CO +1
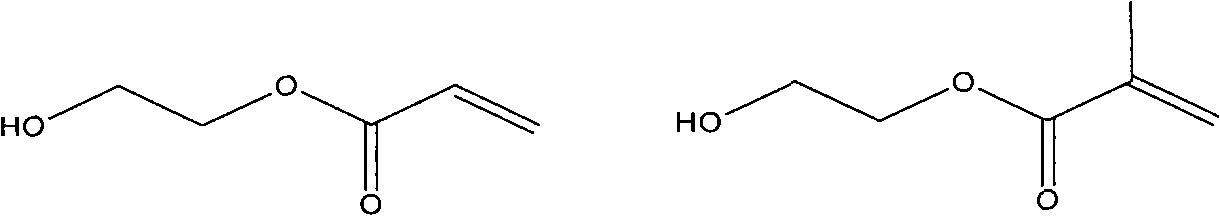
Method for preparing adamantyl unsaturated ester
InactiveCN101870649AAvoid generatingThe synthesis steps are simplePreparation from carboxylic acid halidesCoatingsOrganic solventEvaporation
The invention relates to a method for preparing adamantyl-containing unsaturated ester. The method for preparing the monomer comprises the following steps of: simultaneously adding hydroxyl acrylate and tertiary amine into organic solvent for stirring; dissolving adamantanecarboxylic acid chloride in the organic solvent, and slowly dripping the solvent in mixed solution to react at the temperature of between 0 and 5 DEG C; continuously stirring the solution for 5 hours after 3-hour dripping is completed; standing the mixture over-night; filtering the solution; and washing and drying the solution and performing rotary evaporation to obtain the adamantyl unsaturated ester. The preparation method has the advantages of simple and convenient experiment steps, easily controlled reaction process and reduction of possibility of generating side products, and easy realization of industrialization.
Owner:HANGZHOU INST OF ADVANCED MATERIAL BEIJING UNIV OF CHEM TECH
Insecticidal and antiviral foliar fertilizer and preparation method thereof
InactiveCN109053287AHigh yieldHigh purityAlkali orthophosphate fertiliserAmmonium orthophosphate fertilisersFormateCerium
The invention discloses insecticidal and antiviral foliar fertilizer and a preparation method thereof. The preparation method comprises preparation of rimantadine hydrochloride, preparation of a saponin extract and preparation of foliar fertilizer, wherein the rimantadine hydrochloride is prepared by the following steps: carrying out heating reflux reaction on adamantanecarboxylic acid and thionylchloride, extracting benzene, slowly adding a benzene extract liquor into a three necked bottle containing trimethylaluminum and cerium formate for reaction, and then treating with ice water to obtain adamantane methyl ketone; adding hydroxylamine hydrochloride, alkali and alcohol for oximation into ketoxime, catalyzing by platinum-carbon for hydrogenation reduction, and then acidizing to form salt by hydrogen chloride gas, so as to obtain rimantadine hydrochloride. The preparation method has the beneficial effects that by preparing rimantadine hydrochloride with trimethylaluminum and ceriumformate, a previous route for synthesizing adamantane methyl ketone is changed, and mild reaction conditions, fewer by-products and high product yield are achieved; the foliar fertilizer prepared withrimantadine hydrochloride not only has insecticidal, antibacterial and antiviral effects, but also is capable of quickly supplementing foliar nutrition.
Owner:赵旭萌
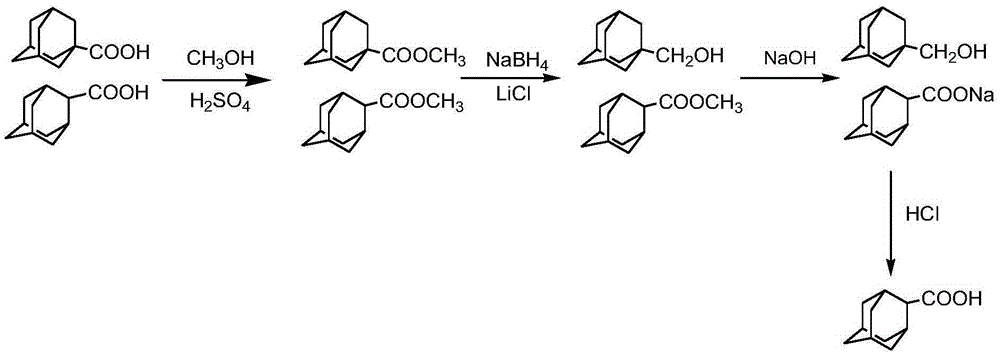
2-adamantanecarboxylic acid and 1-adamantane methanol separation and purification method
InactiveCN105294428AEasy to controlHigh purityPreparation from carboxylic acid saltsOxygen-containing compound preparationLithium chloridePurification methods
The present invention discloses a 2-adamantanecarboxylic acid and 1-adamantane methanol separation and purification method comprising the following steps: 1-adamantanecarboxylic acid and 2-adamantanecarboxylic acid are dissolved in methanol to obtain a liquid mixture A, esterification of the liquid mixture A is first performed to obtain a liquid mixture B, sulfuric acid with the mass fraction of not less than 98% is used as a catalyst of the esterification reaction, the mass ratio of the catalyst and the mixed adamantanecarboxylic acid is 2 to 4:10, the reaction time is 30-60min, and the reaction temperature is 55 to 70 DEG C; reduction reaction of the obtained liquid mixture B is performed to obtain a liquid mixture C, sodium borohydride and lithium chloride are used as reducing agents, the reaction time is 30-120min, and the reaction temperature is 70-85 DEG C; the resulting liquid mixture C is added into a NaOH solution for saponification reaction, an aqueous layer B and an organic layer C are obtained by separation; the separated organic layer C is further purified to obtain 1-adamantane methanol; the separated aqueous layer B is further acidified to obtain 2-adamantanecarboxylic acid. The method is simple and easy to control, and two high-purity 1-adamantane methanol and 2-adamantanecarboxylic acid can be obtained simultaneously.
Owner:ZHEJIANG NORMAL UNIVERSITY
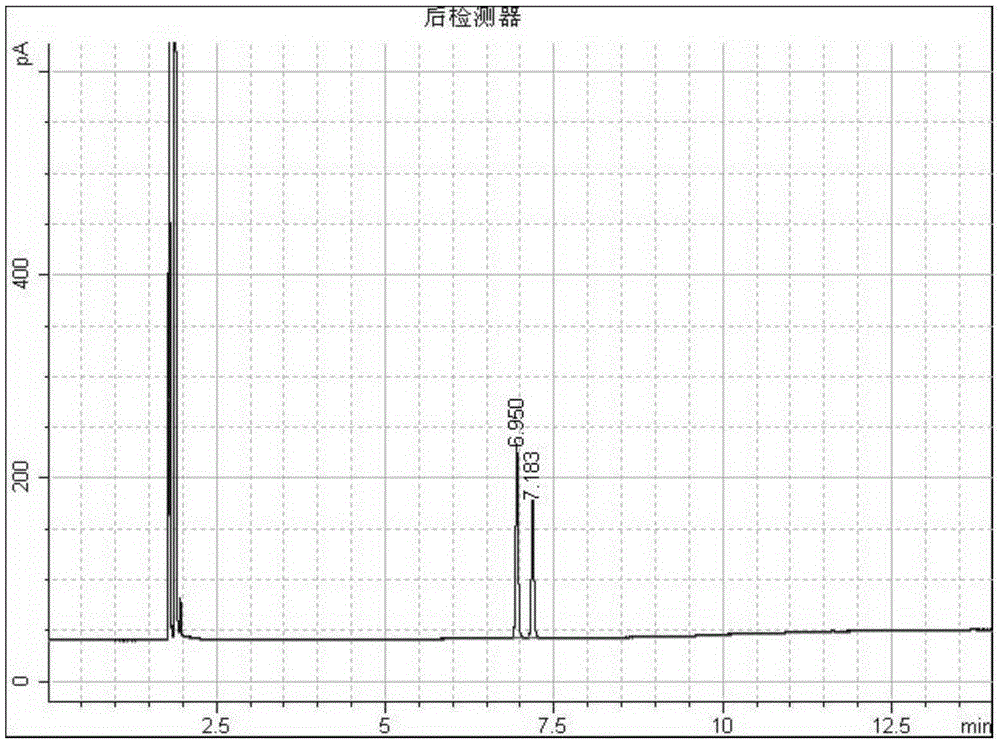
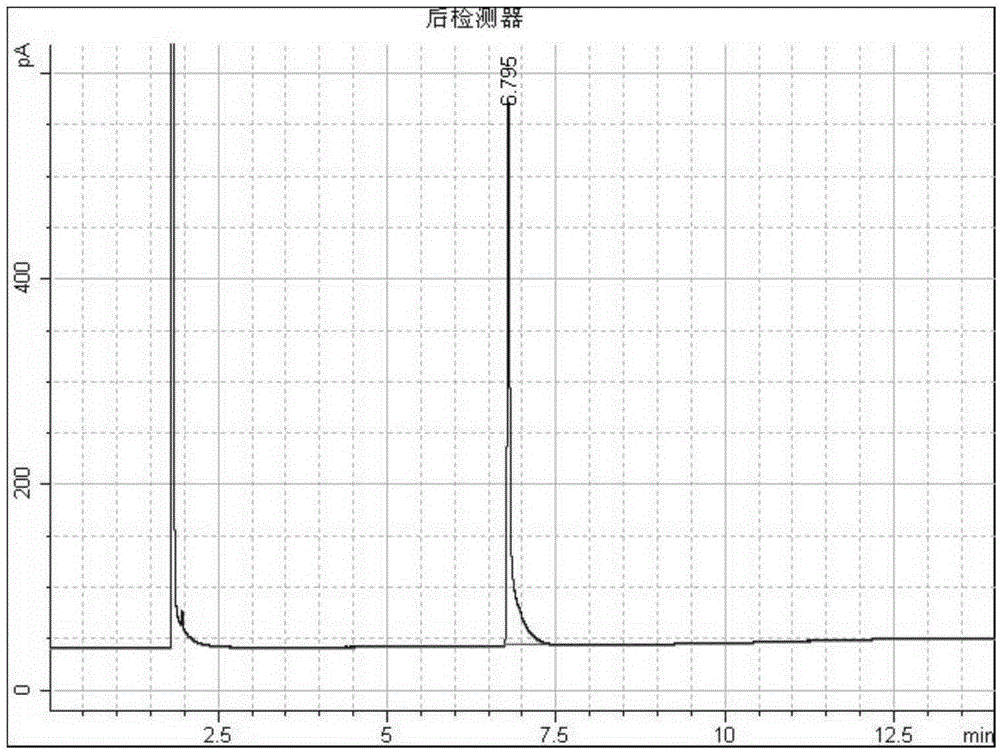
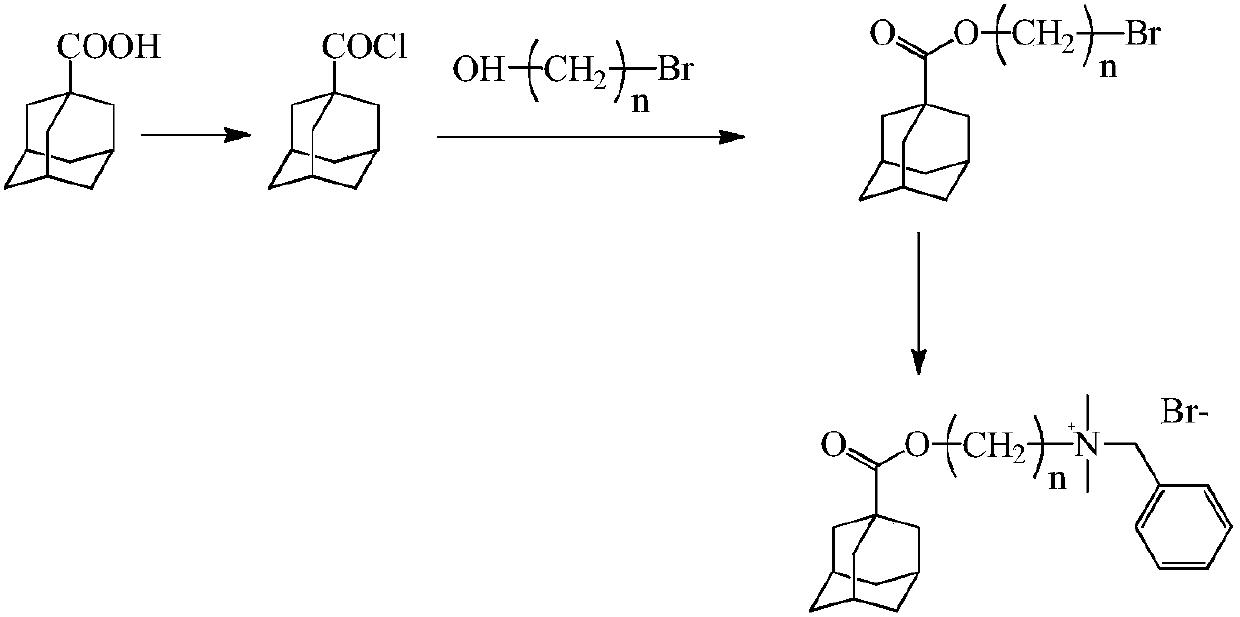
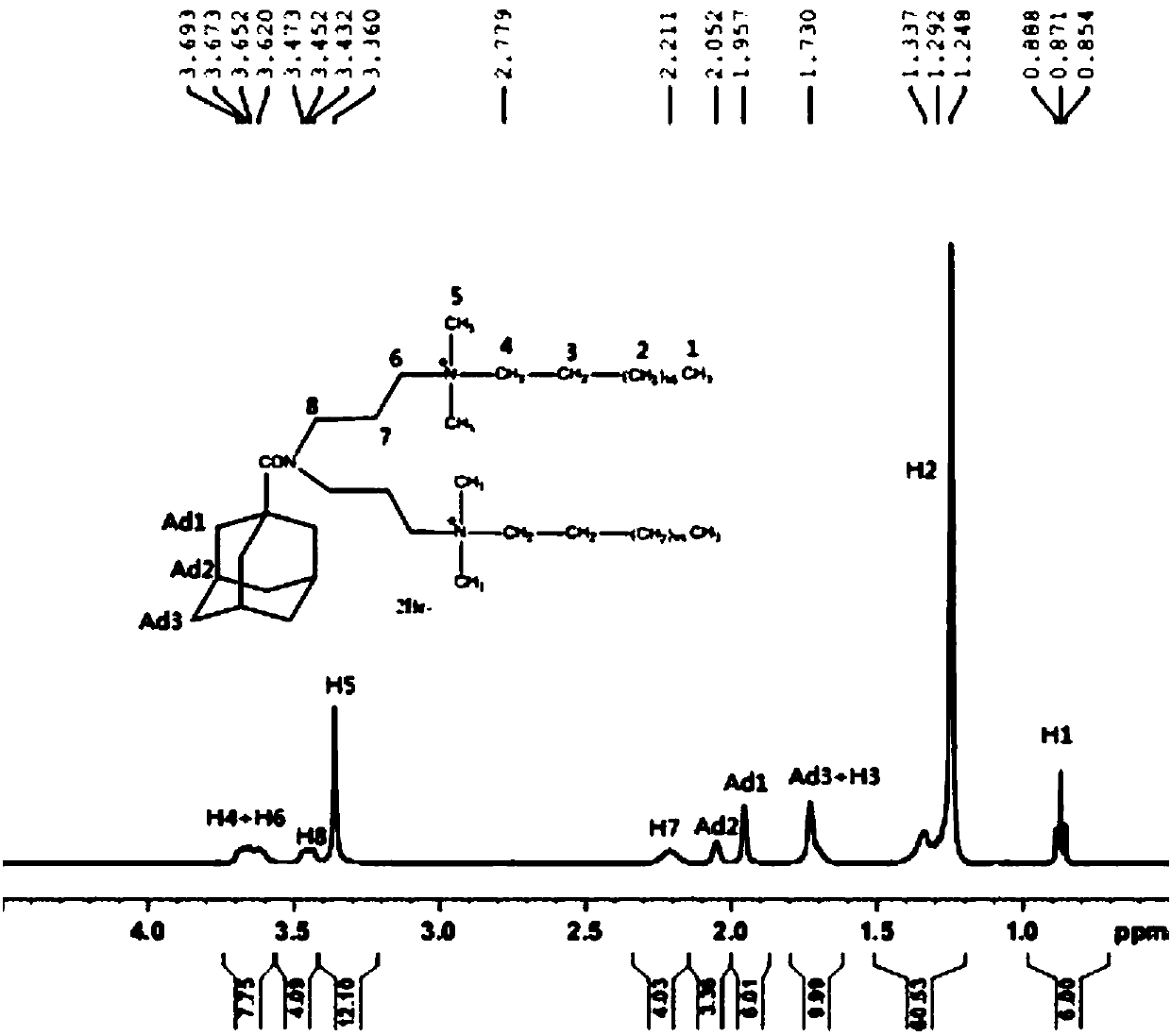
Adamantane-containing quaternary ammonium salt ionic liquid and preparation method thereof
InactiveCN109879765AEnhance amphipathicWill not affect the subject-guest combinationOrganic compound preparationTransportation and packagingQuaternary ammonium cation1-adamantanecarbonyl chloride
The invention relates to an adamantane-containing quaternary ammonium salt ionic liquid and a preparation method thereof. The structural formula of the adamantane-containing quaternary ammonium salt ionic liquid designed and prepared by the invention is shown in the specification, and in the formula, n is equal to 6, 9 or 11. According to the adamantane-containing quaternary ammonium salt ionic liquid disclosed by the invention, 1-adamantanecarboxylic acid is used as a raw material, and the ionic liquid is prepared by the following three steps: firstly, carrying out acylating chlorination reaction on 1-adamantanecarboxylic acid and thionyl chloride to synthesize 1-adamantanecarbonyl chloride, carrying out esterification reaction on the 1-adamantanecarbonyl chloride and OH(CH2)nBr, and thencarrying out quaternization with N,N-dimethylbenzylamine to form the adamantane-containing quaternary ammonium salt ionic liquid. The adamantane-containing quaternary ammonium salt ionic liquid obtained in the invention has the characteristics of mild reaction conditions, easily available raw materials and high product purity, and has potential application prospects in the fields of supramolecular chemistry, catalysis, batteries, nano materials and the like.
Owner:HEZHOU UNIV
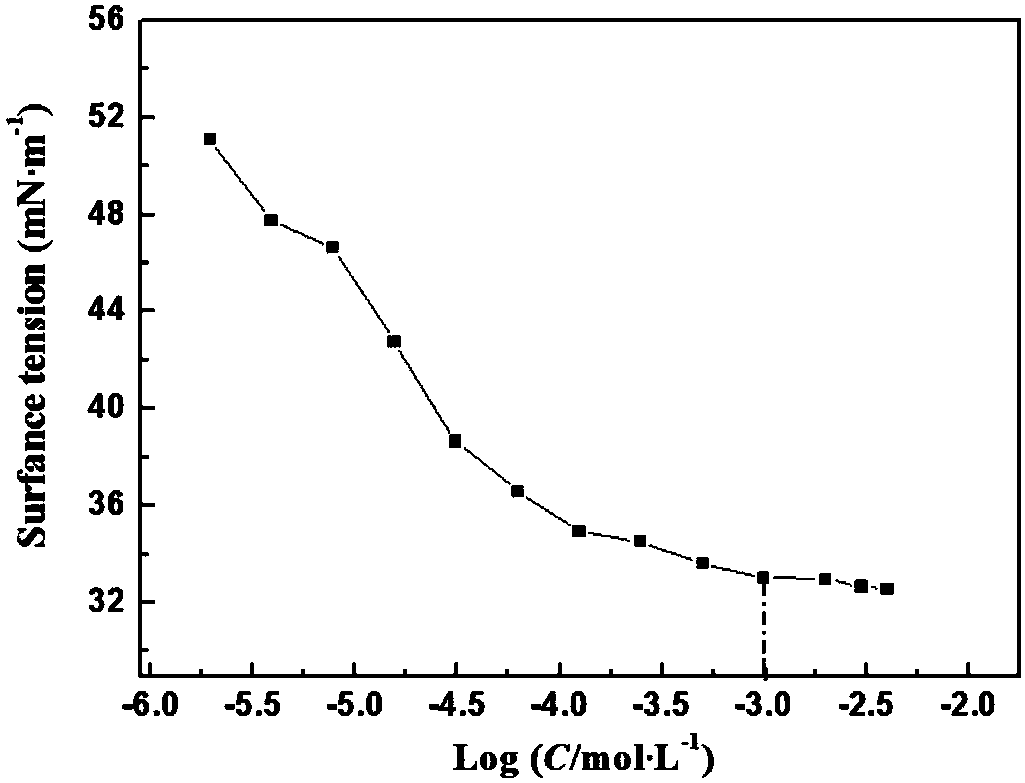
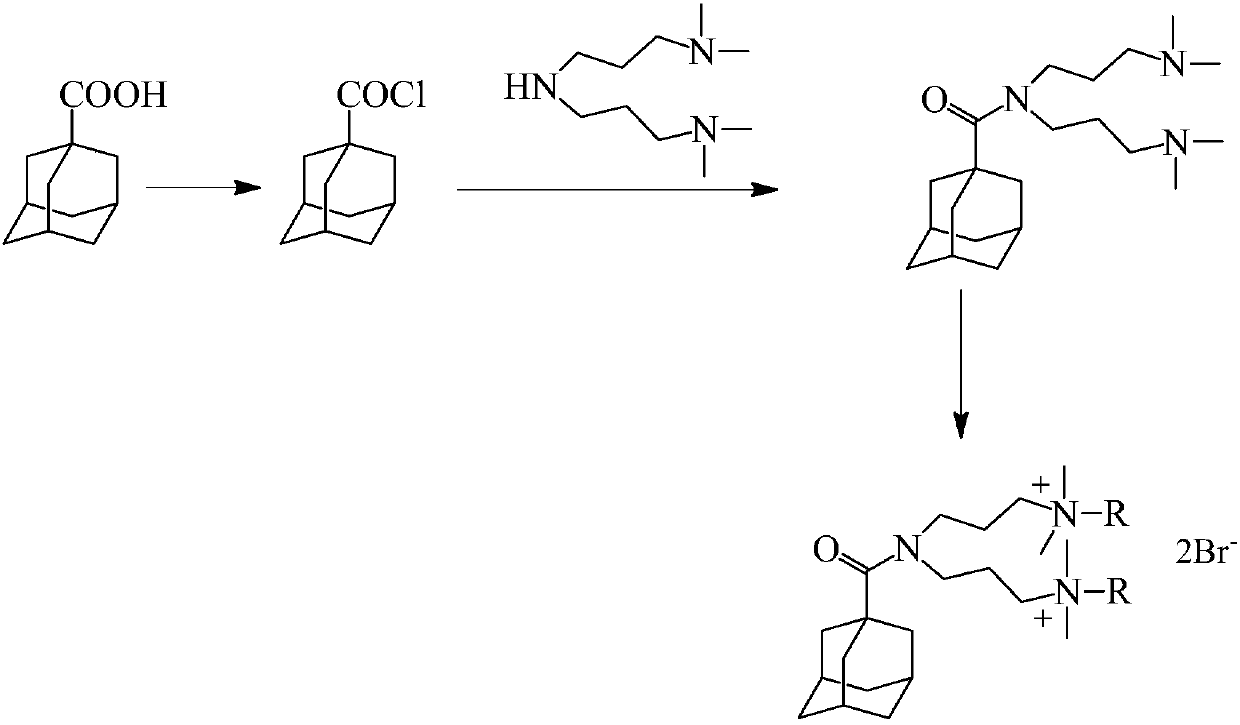
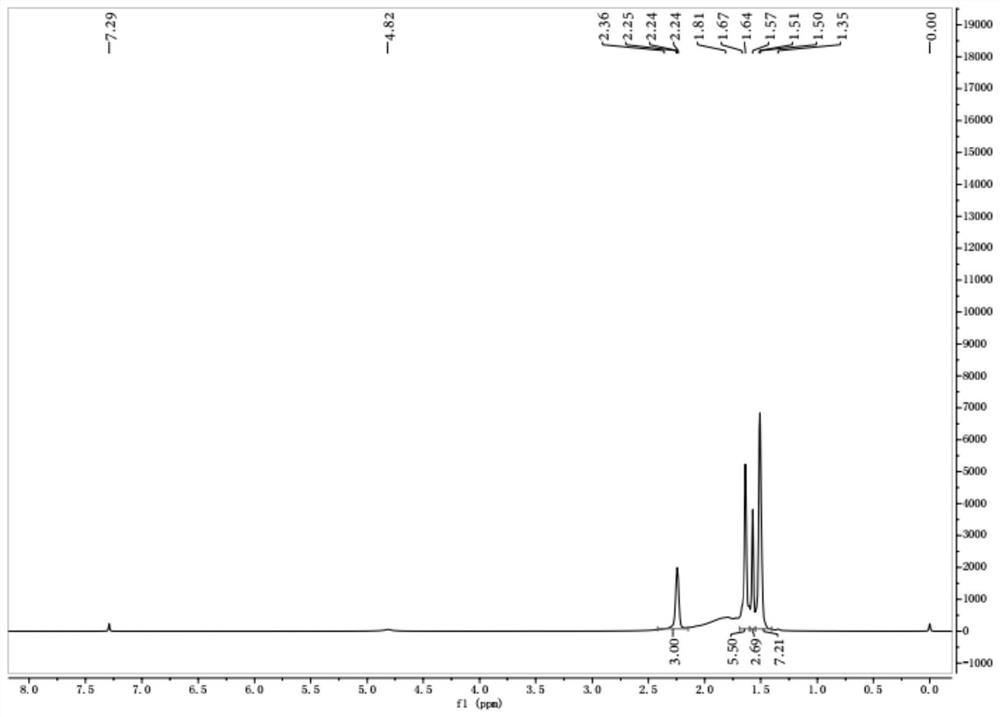
Adamantane-containing Gemini surfactant and synthesis method thereof
ActiveCN109836349AEnhance amphipathicWill not affect the subject-guest combinationOrganic compound preparationTransportation and packagingAlkaneSynthesis methods
The invention belongs to the technical field of organic chemical industry surface activity, and particularly relates to synthesis of an adamantane-containing Gemini surfactant, wherein the adamantane-containing Gemini surfactant has a structural formula defined in the specification, R represents CnH2n+1, and n is 12,16 or 18. According to the present invention, the Gemini surfactant is obtained byusing 1-adamantanecarboxylic acid as a raw material through a three-step reaction, wherein the three-step reaction comprises that 1-adamantanecarboxylic acid and thionyl chloride are subjected to anacyl chlorination reaction to synthesize 1-adamantanecarbonyl chloride, the 1-adamantanecarbonyl chloride and 3,3'-iminobis(N,N-dimethylpropylamine) are subjected to an amidation reaction to prepare akey intermediate bis(N,N-dimethylpropylamine)adamantane-1-formamide, and finally the intermediate and a long chain brominated alkane are subjected to quaternization to form the adamantane-containingGemini surfactant. According to the present invention, the obtained adamantane-containing Gemini surfactant has characteristics of mild reaction condition, easily available raw materials and simple and easy-performing operation, and has potential application prospects in the fields of supramolecular chemistry, nanometer materials, special washing and the like.
Owner:HEZHOU UNIV
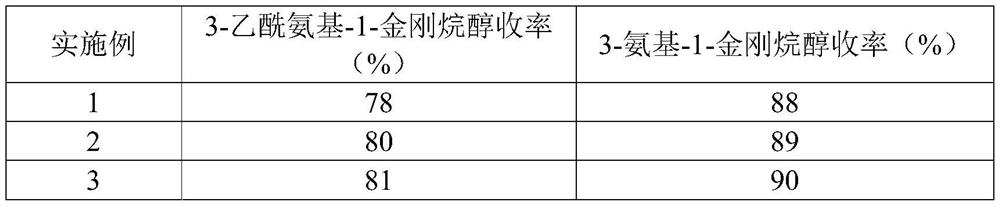
3-amino-1-adamantanol and synthesis method thereof
PendingCN112694410ASimple stepsEasy to operateOrganic compound preparationCarboxylic acid amide separation/purificationAlcoholOrganic synthesis
The invention relates to the technical field of organic synthesis, in particular to 3-amino-1-adamantanol and a synthesis method thereof. The synthesis method of 3-amino-1-adamantanol comprises the following steps of: mixing 3-acetyl amino-1-adamantanol, strong base and an alcohol solvent, heating to 100-200 DEG C in a closed environment, reacting for 5-10h, cooling, crystallizing, filtering, washing the filter cake with the alcohol solvent, merging the filtrate, and distilling to remove the solvent, thereby obtaining the 3-amino-1-adamantanol. The synthesis method of 3-amino-1-adamantanol provided by the invention has the characteristics of simple steps, easiness in operation, high yield, high efficiency and suitability for industrial production; and the used raw material is adamantane, and special adamantane derivatives such as amantadine and adamantanecarboxylic acid are not needed as raw materials, so that the production cost is further reduced.
Owner:广东仁康达材料科技有限公司
Method for synthesizing 3-amino-1-adamantanol
InactiveCN101747212BEasy to operateHigh yieldOrganic compound preparationAmino-hyroxy compound preparationBromateCurtius rearrangement
The invention discloses a method for synthesizing 3-amino-1-adamantanol. The structural formula of the 3-amino-1-adamantanol is as follows. Using adamantanecarboxylic acid as starting material, the method synthesizes the 3-amino-1-adamantanol according to the following reaction steps: bromate 3-amino-1-adamantanol is synthesized by way of bromination, modified curtius rearrangement and hydrolyzation, and finally, bromate is removed by a alkaline liquor treatment method, so that the 3-amino-1-adamantanol is obtained. The invention has the advantages of simple and easy operation, available materials, less reaction steps and high product yield.
Owner:GUANGDONG UNIV OF TECH
One-step synthesis of monodisperse AU-CU nanocubes
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF COMMERCE THE NAT INST OF STANDARDS & TEHCNOLOGY
Polymerizable nonionic surfactant containing adamantane structure and preparation method thereof
PendingCN114751829AGood water solubilityExpand the categoryPreparation from carboxylic acid halidesOrganic compound preparationPolymer sciencePtru catalyst
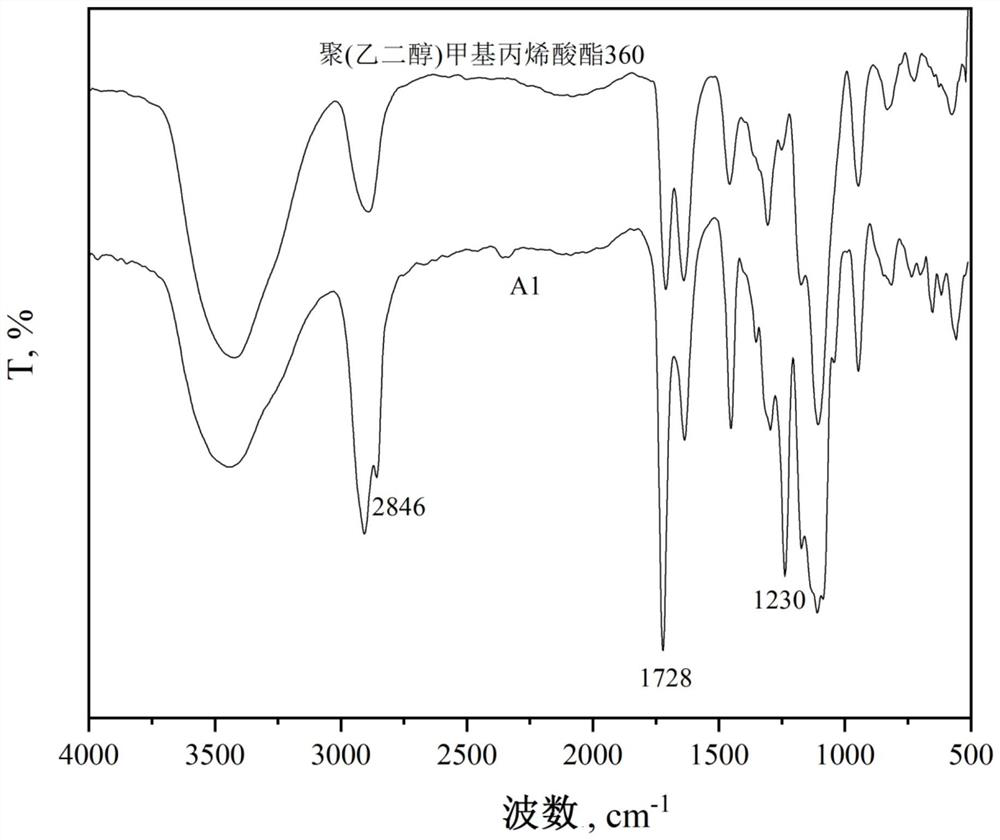
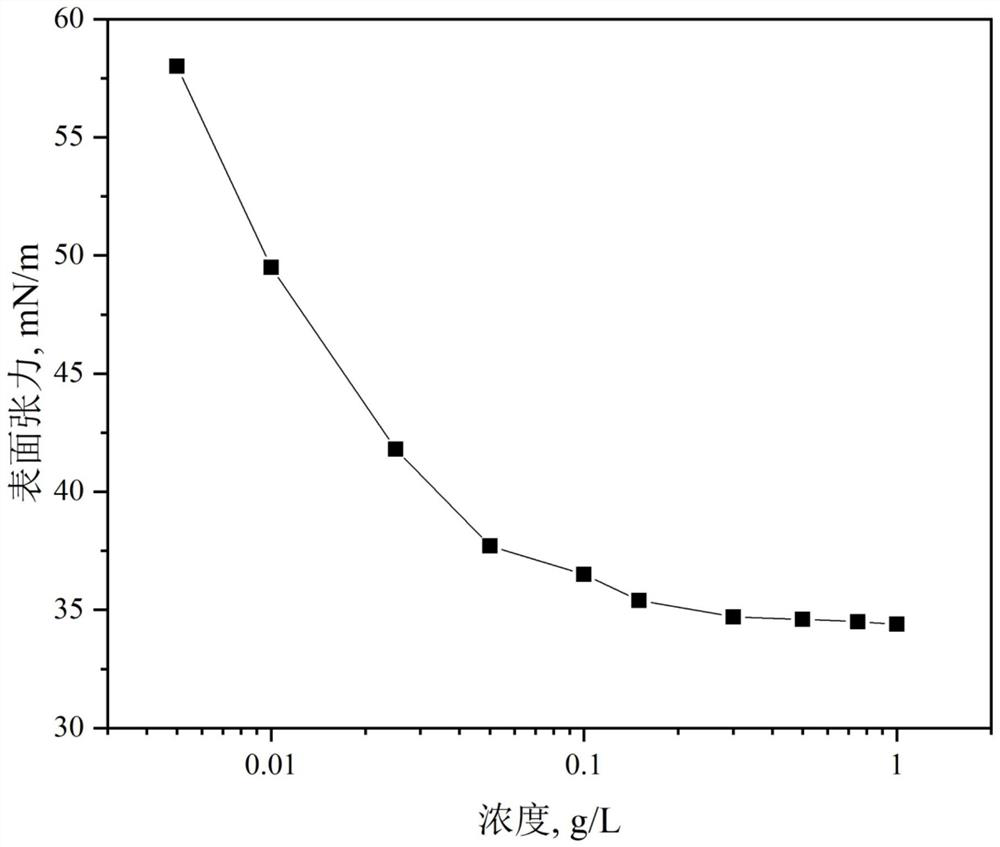
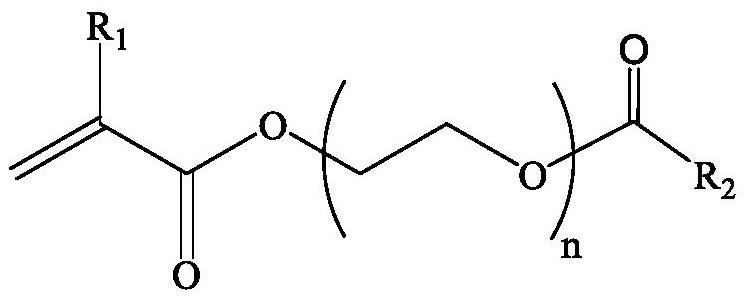
The invention provides a polymerizable nonionic surfactant containing an adamantane structure and a preparation method of the polymerizable nonionic surfactant. The synthesis method of the polymerizable nonionic surfactant comprises the following step: carrying out esterification reaction on adamantyl acyl chloride and polyethylene glycol acrylate in an organic solvent under the condition that organic alkali is used as an acid-binding agent, so as to obtain the polymerizable nonionic surfactant. Or adamantane carboxylic acid and polyethylene glycol acrylate are subjected to esterification reaction in an organic solvent by using a catalyst. A polymerizable double-bond structure is contained on a molecular terminal group of a product obtained after reaction, the product can be applied to polymerization reaction to serve as a hydrophobic monomer, and the product has surface activity.
Owner:PETROCHINA CO LTD
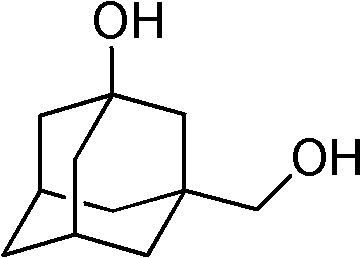
The synthetic method of 1-hydroxyl-3-hydroxymethyladamantane
ActiveCN103058826BHigh yieldEase of industrial productionOrganic compound preparationHydroxy compound preparationBranHydroxymethyl
Owner:XUZHOU B&C CHEM CO LTD
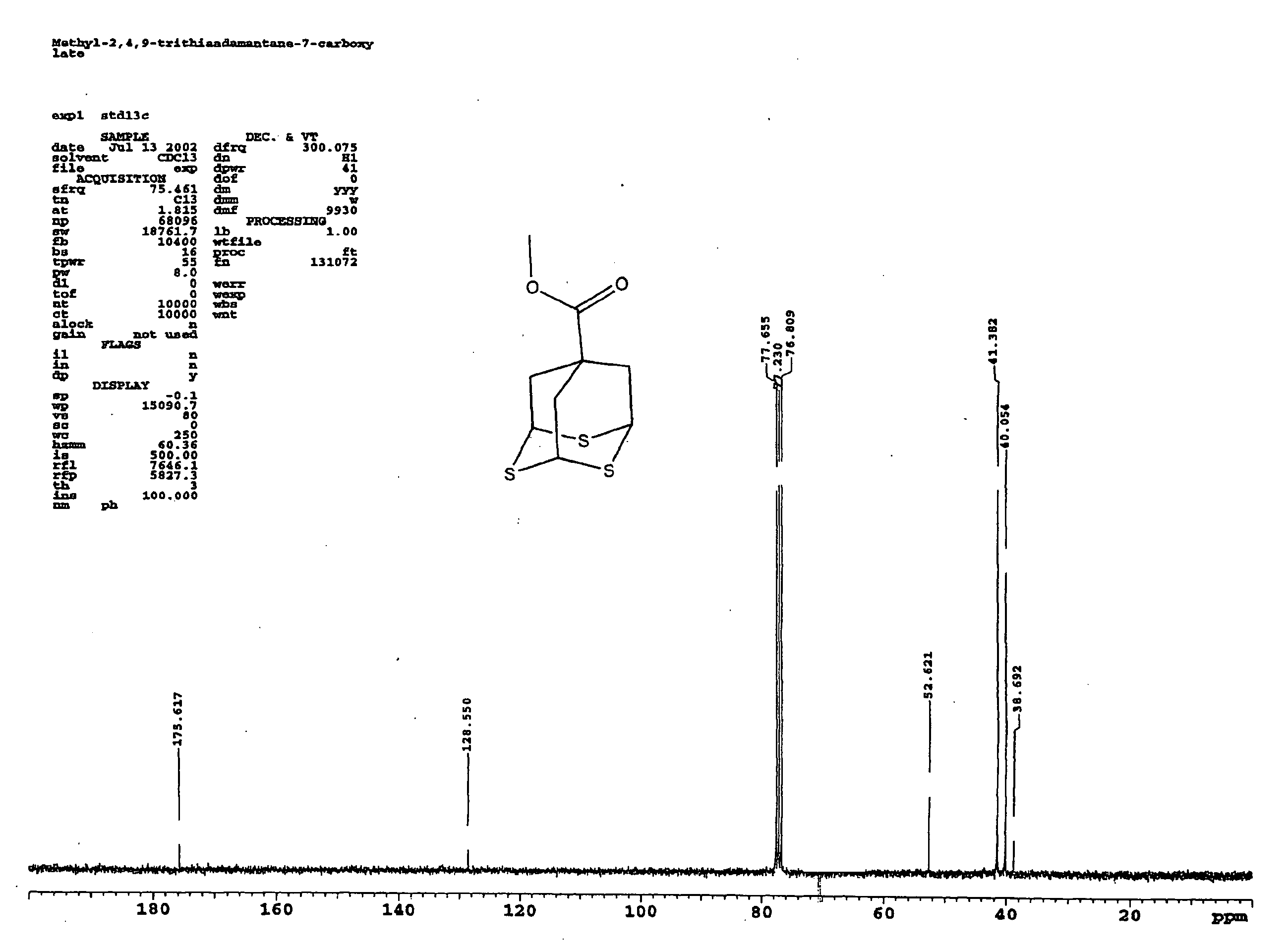
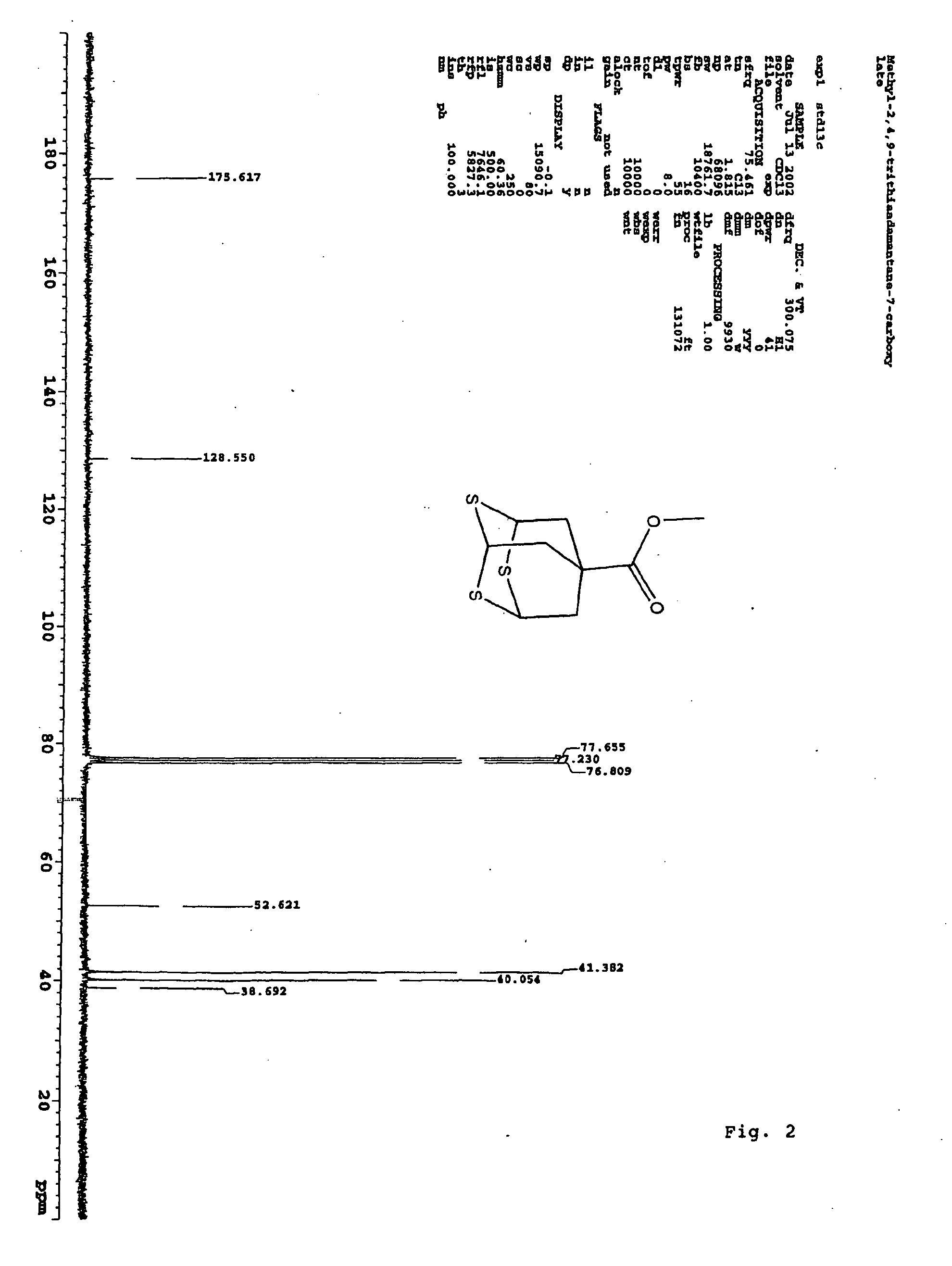
Methyl 2,4,9-trithiaadamantane-7-carboxylate
Methyl 2,4,9-trithiaadamantane-7-carboxylate and a method for its manufacture is disclosed. The method reacts oxidized methyl triallyl acetate with a Lewis acid and a sulphuring agent.
Owner:THE UNIVERSITY OF AKRON +1
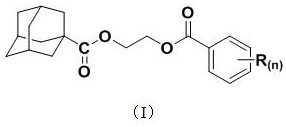
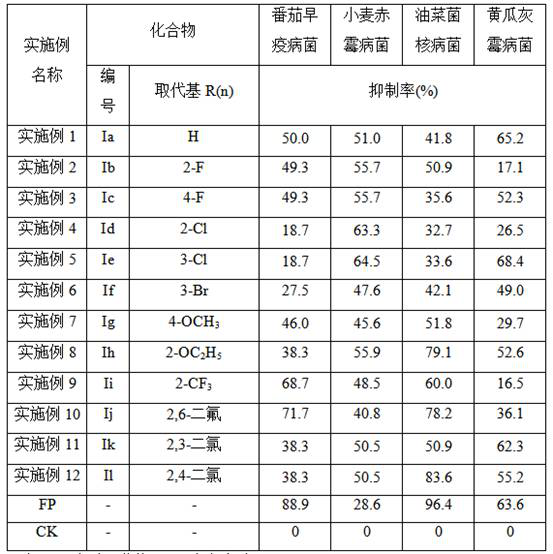
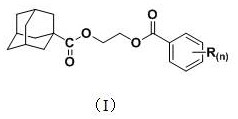
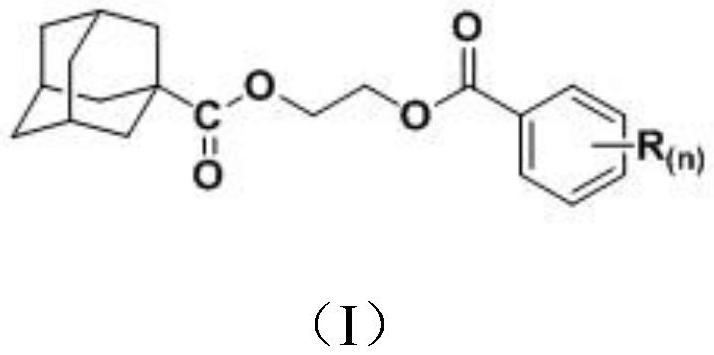
Application of a 1-adamantanecarboxylic acid-2-(substituted benzoyloxy) ethyl ester compound as a fungicide
The invention discloses applications of a 1-adamantanecarboxylic acid-2-(substituted benzoyloxy)ethyl ester compound as bactericides, wherein the 1-adamantanecarboxylic acid-2-(substituted benzoyloxy)ethyl ester compound has a structure represented by a formula (I), H on the benzene ring is mono-substituted or di-substituted with a substituent R or is not substituted, n is an integer of 0-2, preferably 1-2, and represents the number of the substituents R on the benzene ring, the H on the benzene ring is not substituted when n is 0, the H on the benzene ring is mono-substituted by the substituent R when n is 1, the H on the benzene ring is di-substituted by the substituent R when n is 2, the substituents R at different substitution positions are the same or different, and the substituents Rare hydrogen, C1-C3 alkoxy, C1-C2 haloalkyl, and halogen. According to the present invention, the 1-adamantanecarboxylic acid-2-(substituted benzoyloxy)ethyl ester compound particularly has good inhibition effect on Alternaria solani, Fusarium graminearum, Sclerotinia sclerotiorum, Botrytis cinerea and other fungi.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of 1-adamantyl methy ketone
InactiveCN109809975ALow costEasy to operateOrganic compound preparationCarbonyl compound preparation1-adamantanecarbonyl chlorideKetone
The invention belongs to the field of preparation of chemical intermediates, and particularly relates to a preparation method of 1-adamantyl methy ketone. The preparation method comprises the following steps of step 1, enabling adamantane and liquid bromine to react, so as to obtain 1-bromoadamantane; step 2, using concentrated sulfuric acid as a catalyst, adding the 1-bromoadamantane and n-hexane, and dripping formic acid, so as to obtain 1-adamantanecarboxylic acid; step 3, enabling the 1-adamantanecarboxylic acid and thionyl chloride to react, heating and refluxing, extracting by benzene, and directly applying the extracting liquid for the reaction in next step; step 4, dripping a mixed solution of benzene, diethyl malonate and anhydrous ethyl alcohol into a mixed solution of magnesiumpowder, iodine, anhydrous ethyl alcohol and benzene, dripping a benzene solution of 1-adamantanecarbonyl chloride, extracting by benzene, drying and distilling, so as to obtain 2- adamantanecarbonyl diethyl malonate; step 5, adding glacial acetic acid, water and concentrated sulfuric acid into the residue. The preparation method has the advantage that the yield rate is high.
Owner:PANJIN GELIN KAIMO TECH CO LTD
A kind of preparation method of 2-(2-alkylphenoxy)pyridine derivatives
InactiveCN107043350BHigh puritySynthetic raw materials are readily availableOrganic chemistryCyclohexanecarboxylic acidGlycol synthesis
A preparation method of a 2-(2-alkylphenoxy)pyridine derivative adopts a reaction of a 2-phenoxypyridine derivative and a primary bromoalkane compound. The method concretely comprises the following steps: directly adding the 2-phenoxypyridine derivative, the primary bromoalkane compound, a catalyst, an additive, an alkali and a solvent into a reaction device, stirring and heating the above added substances until the temperature is 90-130 DEG C, carrying out a reaction for 18-36 h, and separating the obtained product to obtain the 2-(2-alkylphenoxy)pyridine derivative, wherein the catalyst is dichloro(p-cymene)ruthenium dimer; the alkali is potassium carbonate or lithium carbonate; the additive is 1-adamantanecarboxylic acid or naphthenic acid; and the solvent is benzene or DMF or ethylene glycol dimethyl ether. The method has the advantages of easiness in obtaining of synthesis raw materials, simple process, and very good specificity of the reaction.
Owner:ANYANG NORMAL UNIV
Synthesis method of 3-hydroxy-1-adamantanecarboxylic acid
InactiveCN109704948ALow costEasy to operateOrganic compound preparationCarboxylic compound preparationOrganic synthesisSynthesis methods
The invention belongs to the field of organic synthesis and particularly relates to a synthesis method of 3-hydroxy-1-adamantanecarboxylic acid. In the method, with 1-adamantanecarboxylic acid being araw material and triethyl benzyl ammonium chloride (TEBAC) being a phase transfer catalyst, the 3-hydroxy-1-adamantanecarboxylic acid is prepared under alkaline conditions through hydroxylation withKMnO4. The new preparation method not only is reduced in cost and protects environment, but also is simple in operation and convenient in post-treatment. The method is high in yield, is simple in synthesis and is suitable for industrial production.
Owner:赵明
A kind of 1-adamantanecarboxylic acid-2-(substituted benzoyloxy) ethyl ester compound and its synthetic method and application
ActiveCN110483284BEasy to makeExhibit antitumor activityPreparation from carboxylic acid halidesAntineoplastic agentsMeth-Alkoxy group
The invention discloses a 1-adamantanecarboxylic acid-2-(substituted benzoyloxy)ethyl ester compound and its synthesis method and application. The structural formula of the ester compound is shown in formula (I): in formula (I), H on the benzene ring is monosubstituted, disubstituted or unsubstituted by substituent R; n is an integer of 0~2, preferably 1~2 An integer of 2, n represents the number of substituent R on the benzene ring; when n=0, it means that the H on the benzene ring is not substituted; when n=1, it means that the H on the benzene ring is monosubstituted by a substituent R; n When =2, it means that the H on the benzene ring is disubstituted by the substituent R, and the substituent R in different substitution positions is the same or different; the substituent R is hydrogen, C1~C3 alkoxy, C1~C2 haloalkyl, halogen , preferably hydrogen, methoxy, ethoxy, trifluoromethyl, F, Cl or Br. The synthesis method of the 1-adamantanecarboxylic acid-2-(substituted benzoyloxy)ethyl ester compound of the present invention is simple, and it exhibits certain antitumor activity.
Owner:ZHEJIANG UNIV OF TECH
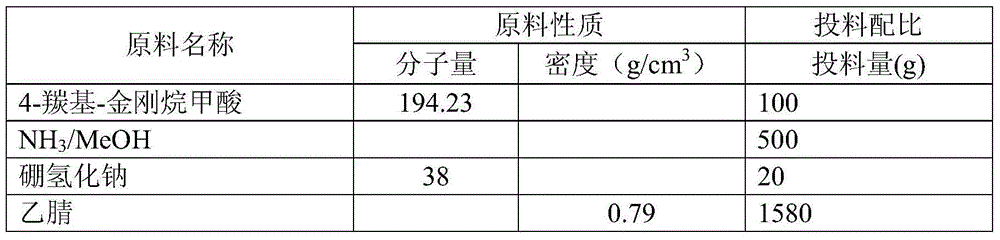
Method for preparing 4-amino-adamantanecarboxylic acid
InactiveCN104926672ANo pollution in the processSignificant technological progressOrganic compound preparationAmino-carboxyl compound preparationOrganic solventHydrogen
The invention provides a method for preparing 4-amino-adamantanecarboxylic acid. 4-carbonyl-adamantanecarboxylic acid, five to ten N of NH<3> / MeOH solutions and sodium borohydride are firstly weighed, the 4-carbonyl-adamantanecarboxylic acid and the NH<3> / MeOH solutions are added into a reaction vessel and stirred at room temperature, reaction liquid is cooled to -5 DEG C to 2 DEG C, the sodium borohydride is added, stirring is carried out for two to six hours, the reaction liquid is spin-dried, water is added for dissolution, stirring is carried out, PH is adjusted to five to six through hydrochloric acid, stirring continues, the reaction liquid is extracted two to five times through first organic solvents, and the first organic solvents are combined. After spin-drying, second organic solvents and water are added into solids for dissolution, stirring is carried out for four to ten hours at room temperature, suction filtration is carried out, and the 4-amino-adamantanecarboxylic acid is obtained after solid products are dried. No high-pressure vessel is adopted, reactions are carried out in a common vessel, no noble metal catalyst is adopted, cost is lowered, no hydrogen gas reduction is adopted, high operability is achieved, and the industrial application prospect is achieved.
Owner:上海博康精细化工有限公司
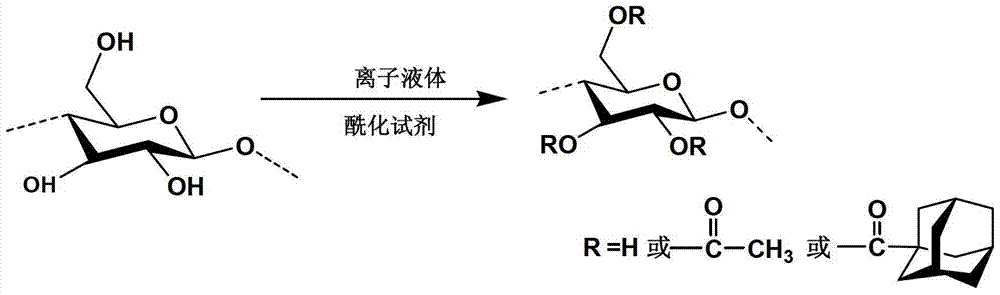
A cellulose hybrid, preparation method and its application
ActiveCN104140468BHigh mechanical strengthHigh selectivitySemi-permeable membranesPolymer scienceCellulose acetate
The invention discloses a cellulose mixed ester, which is a cellulose acetate adamantane carboxylate mixed ester having an acetate group and an adamantane carboxylate group, and its structural formula is: wherein, the degree of substitution of the acetate group is greater than 0 to 3, the degree of substitution of the adamantanecarboxylic acid group is greater than 0 to 2.25. The above-mentioned cellulose mixed ester introduces acetate groups and adamantane carboxylate groups into its cellulose main chain, and the material has high solubility, good film-forming properties, and a controllable degree of substitution. The cellulose mixed ester can be synthesized by one-step method to obtain adamantane formate cellulose mixed ester, and the synthesis is simple, efficient and stable.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com