Synthetic method for rimantadine
A technology of rimantadine and its synthesis method, which is applied in the preparation of amino-substituting functional groups, organic chemistry, etc., can solve the problems of high cost and high cost, and achieve the effects of low synthesis cost, high yield and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
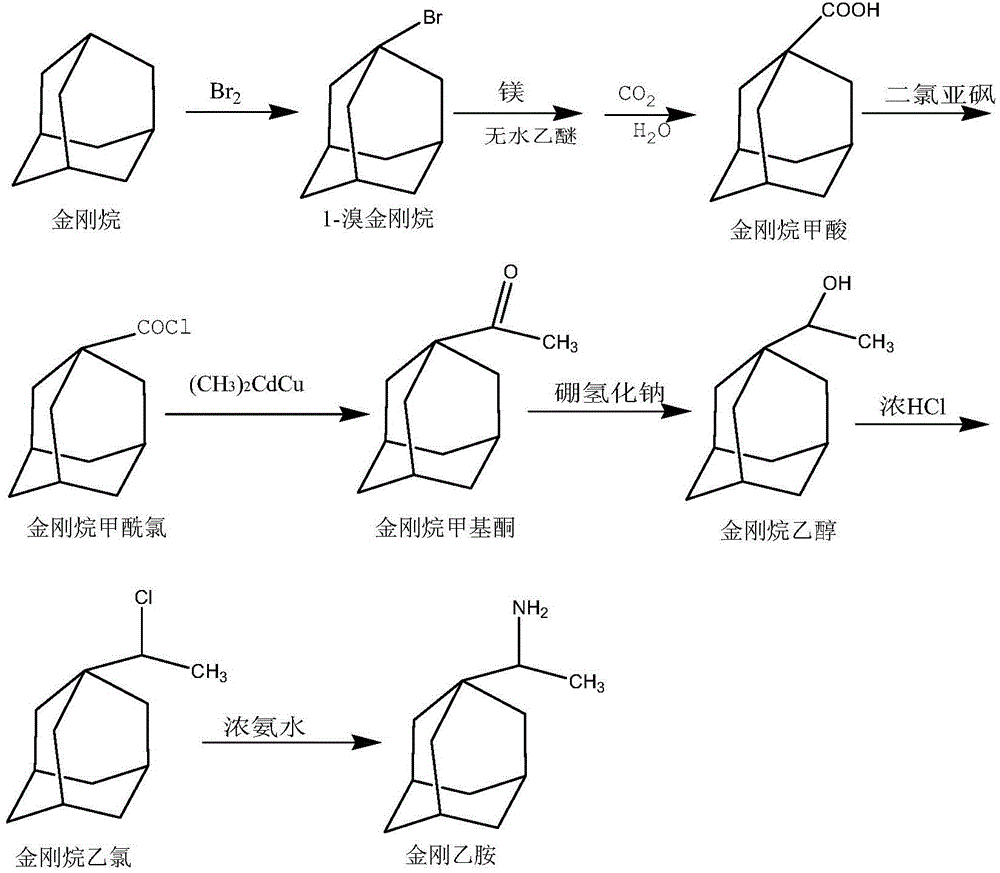
[0028] The present embodiment prepares rimantadine according to the following steps:
[0029] (1) Preparation of 1-bromoadamantane:
[0030] Add 5g of adamantane and 4mL of liquid bromine in sequence in the flask, heat to 85°C for 6h, then heat in an oil bath at 113°C for 3h, let stand for 10h, distill off excess liquid bromine, and then use 3mL of saturated sodium bisulfite to reduce the reaction The remaining liquid bromine in the liquid was filtered, the filter cake was washed to neutrality, dried at 100°C for 3 hours, and recrystallized by methanol to obtain 7.2 g of light yellow crystals, namely 1-bromoadamantane, with a yield of 92.4%.
[0031] (2) Preparation of adamantanecarboxylic acid:
[0032] Add 2g of 1-bromoadamantane, 20mL of anhydrous ether, excess magnesium powder and 0.1g of iodine into a 50mL three-necked flask, replace with nitrogen 3 times, react for 2h, until the magnesium powder does not decrease, and pass CO into the reaction solution 2 At the end of ...
Embodiment 2
[0040] The present embodiment prepares rimantadine according to the following steps:
[0041] (1) Preparation of 1-bromoadamantane:
[0042] Add 3g of adamantane and 3mL of liquid bromine successively into a 50mL three-necked flask, heat to 85°C for 6h, then heat in an oil bath at 113°C for 3h, let it stand for 10h, distill off the remaining liquid bromine, and then use 3mL of saturated Sodium bisulfate reduced the remaining liquid bromine in the reaction solution, filtered, washed the filter cake to neutrality, dried at 100°C for 3 hours, and recrystallized through methanol to obtain 4.30 g of light yellow crystals, namely 1-bromoadamantane, with a yield of 92.0% .
[0043] (2) Preparation of adamantanecarboxylic acid:
[0044] Add 4g of 1-bromoadamantane, 40ml of anhydrous ether, excess magnesium powder and 0.2g of iodine into a 50mL three-necked flask, replace with nitrogen 3 times, react for 2h until the magnesium powder does not decrease, and pass CO into the reaction s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com