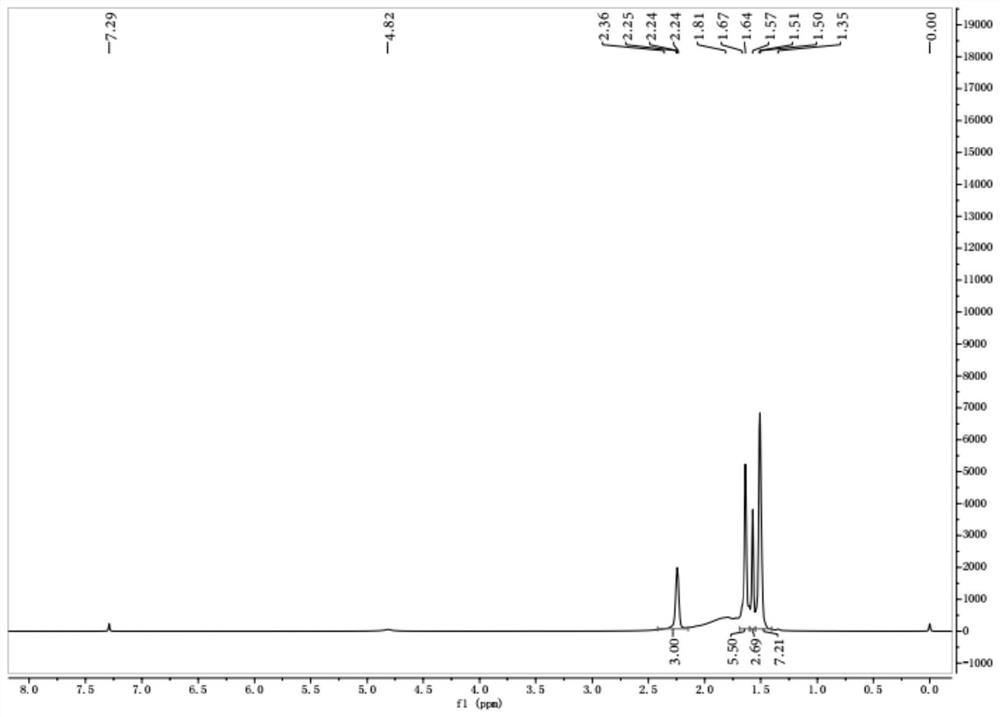
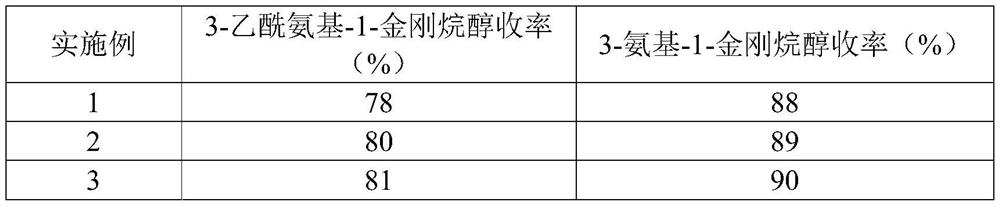
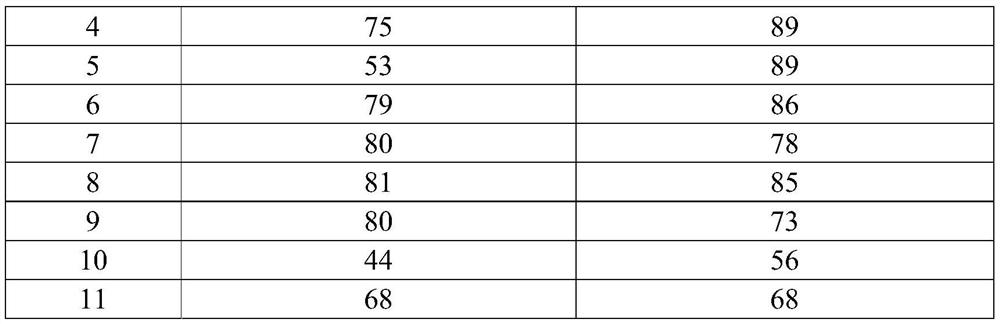
3-amino-1-adamantanol and synthesis method thereof
A technology for adamantanol and a synthesis method, which is applied in the preparation of amino hydroxy compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of difficult industrialization of reaction conditions, low total yield, and many steps, and achieves reduced The effect of high production cost, high yield and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] In one embodiment, the preparation method of 3-acetamido-1-adamantanol comprises: mixing adamantane and strong acid, stirring at 30-100°C for 0.5-3h, and stirring at 30°C within 20-60min Complete the dropwise addition of the nitrile compound, continue the reaction at 40-60°C for 1-10 hours to obtain the reaction liquid, drop the reaction liquid into the mixed extract for extraction, separate the liquid under the condition that the temperature does not exceed 10°C, and use the extract for the aqueous phase -1 and then extract; combine the organic phases, distill, and obtain.
[0031] Preferably, the nitrile compound is fatty nitrile; further preferably, the fatty nitrile is acetonitrile and / or propionitrile; more preferably, the fatty nitrile is acetonitrile.
[0032] In a preferred embodiment, the preparation method of 3-acetamido-1-adamantanol comprises: mixing adamantane and a strong acid, stirring at 30°C for 0.5h, and completing the dropwise addition within 60min be...
Embodiment 1
[0051] Embodiment 1 of the present invention provides a kind of synthetic method of 3-amino-1-adamantanol, and its concrete steps are: (1) adamantane and strong acid are mixed, stirred at 30 ℃ for 3h, under 30 ℃ in 60min The nitrile compound was added dropwise, and the reaction solution was obtained after continuing the reaction at 40°C for 10 hours. The reaction solution was dropped into the mixed extraction liquid for extraction, and the liquid was separated under the condition that the temperature did not exceed 10°C. Perform extraction; combine the organic phases and distill to obtain 3-acetamido-1-adamantanol; (2) mix 3-acetamido-1-adamantanol, strong base, and alcohol solvent, heat up to 160°C in airtight conditions, After reacting for 10 hours, cool and crystallize, filter, wash the filter cake with an alcohol solvent, combine the filtrates, and distill off the solvent to obtain the product.
[0052] The weight ratio of the 3-acetylamino-1-adamantanol, strong base, and ...
Embodiment 2
[0054] Embodiment 2 of the present invention provides a synthetic method of 3-amino-1-adamantanol, the specific steps of which are: (1) mix adamantane with a strong acid, stir at 30°C for 0.5h, and place under 30°C in Complete the dropwise addition of the nitrile compound within 60 minutes, continue the reaction at 60°C for 1 hour to obtain the reaction liquid, drop the reaction liquid into the mixed extract for extraction, separate the liquid under the condition that the temperature does not exceed 10°C, and use the extract-1 for the aqueous phase Then extract; combine the organic phases and distill to obtain 3-acetamido-1-adamantanol; (2) mix 3-acetamido-1-adamantanol, strong base, and alcohol solvent, and heat up to 200°C , After reacting for 5h, cool and crystallize, filter, wash the filter cake with an alcohol solvent, combine the filtrates, and distill off the solvent to obtain that product.
[0055] The weight ratio of the 3-acetylamino-1-adamantanol, strong base, and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com