7-alkyl-N-pyrimidylindoline compound and synthesis method thereof
A compound, indoline technology, applied in the field of preparation of 7-alkyl-N-pyrimidine indoline compounds, can solve problems such as few reports, and achieve the effect of enriching the types of functional grouping reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
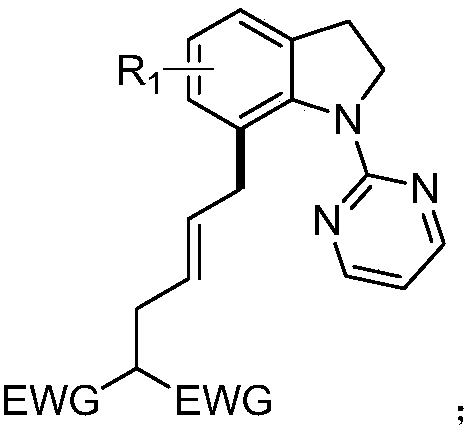
[0024] The structural formula of the compound (E)-2-(4-(1-(2-pyrimidinyl) 7-indolinyl) 2-buten-1-yl) diethyl malonate of the present embodiment is:
[0025]
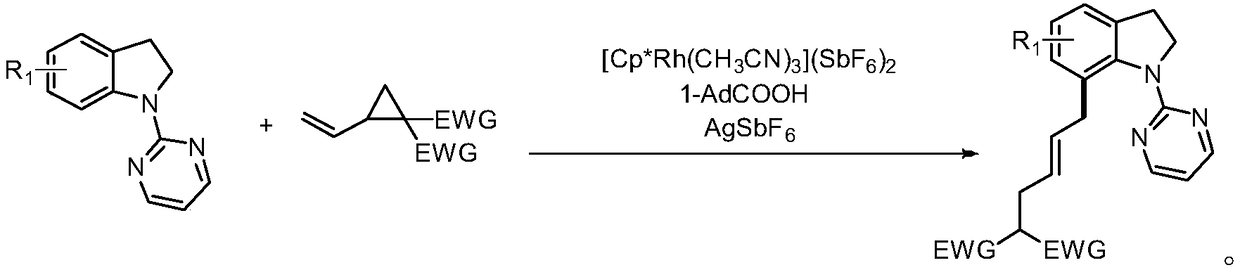
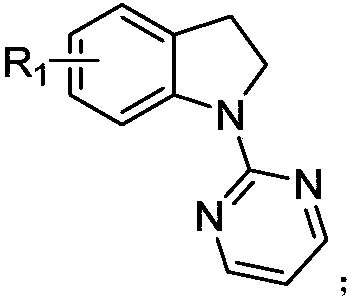
[0026] The preparation method is: under argon protection environment, add 0.2mmol of N-pyrimidine indoline compound, 0.3mmol of ethylene cyclopropane compound, 0.002mmol of [Cp*Rh(CH 3 EN) 3 ](SbF 6 ) 2 , 0.04mmol 1-adamantanecarboxylic acid, 0.02mmol silver hexafluoroantimonate, methanol 2mL, react at 90°C for 12 hours; , eluent: ethyl acetate / petroleum ether gradient elution, the ratio is from 0 / 100 to 100 / 0), and dried to obtain a light yellow oil with a yield of 88%, E / Z=20:1. 1 H NMR (400MHz, CDCl 3 )δ8.46–8.36(m,2H),7.13–6.98(m,3H),6.72–6.64(m,1H),5.69-5.59(m,1H),5.44-5.35(m,1H),4.45– 4.36(m,2H),4.21–4.12(m,4H),3.41–3.19(m,3H),3.05(t,J=7.6Hz,2H),2.66–2.53(m,2H),1.27-1.16( m,6H). 13 C NMR (101MHz, CDCl 3 )δ169.0, 161.3, 157.7, 142.3, 134.7, 131.7, 130.5, 128.2, 127.1, 124.2, 122.3, 112.2, 61.3, 53.2, 52.2...
Embodiment 2
[0028] Compound (E)-2-(4-(2-methyl-1-(2-pyrimidinyl) 7-indolinyl) 2-buten-1-yl) diethyl malonate The structural formula is:
[0029]
[0030] The preparation method is: under argon protection environment, add 0.2mmol of N-pyrimidine indoline compound, 0.3mmol of ethylene cyclopropane compound, 0.002mmol of [Cp*Rh(CH 3 EN) 3 ](SbF 6 ) 2 , 0.02mmol 1-adamantanecarboxylic acid, 0.04mmol silver hexafluoroantimonate, methanol 0.5mL, react at 90°C for 12 hours; Mesh, eluent: gradient elution with ethyl acetate / petroleum ether, the ratio is from 0 / 100 to 100 / 0), and dried to give a light yellow oil with a yield of 78%, E / Z=12:1. 1 H NMR (600MHz, CDCl 3 )δ8.43–8.37(m,2H),7.09–7.00(m,3H),6.67(t,J=4.2Hz,1H),5.64–5.56(m,1H),5.37–5.28(m,1H) ,4.98-4.95(m,1H),4.20-4.07(m,4H),3.48-3.16(m,4H),2.54(m,3H),1.36(t,J=8.7Hz,3H),1.25-1.21 (m,6H). 13 C NMR (101MHz, CDCl 3)δ169.0, 160.8, 157.6, 140.7, 133.5, 131.8, 130.8, 128.3, 126.9, 124.1, 122.9, 112.2, 61.3, 60.4, 52.2, 37.1, 36.9, 31....
Embodiment 3
[0032] Compound (E)-2-(4-(3-methyl-1-(2-pyrimidinyl) 7-indolinyl) 2-buten-1-yl) diethyl malonate The structural formula is:
[0033]
[0034] The preparation method is: under argon protection environment, add 0.2mmol of N-pyrimidine indoline compound, 0.3mmol of ethylene cyclopropane compound, 0.016mmol of [Cp*Rh(CH 3 EN) 3 ](SbF 6 ) 2 , 0.06mmol 1-adamantanecarboxylic acid, 0.02mmol silver hexafluoroantimonate, methanol 0.5mL, react at 90°C for 12 hours; Mesh, eluent: gradient elution with ethyl acetate / petroleum ether, the ratio is from 0 / 100 to 100 / 0), and dried to give a light yellow oil with a yield of 77%, E / Z=15:1. 1 H NMR (400MHz, CDCl 3 )δ8.45–8.37(m,2H),7.08-7.01(m,3H),6.70-6.66(m,1H),5.69-5.60(m,1H),5.43-5.36(m,1H),4.65– 4.56(m,1H),4.21–4.12(m,4H),3.91–3.84(m,1H),3.42–3.19(m,4H),2.59(t,J=7.0Hz,2H),1.28–1.21( m, 9H). 13 C NMR (101MHz, CDCl 3 )δ169.0, 161.5, 157.7, 142.0, 139.9, 131.7, 130.4, 128.3, 127.2, 124.4, 121.1, 112.2, 61.3, 61.0, 52.2, 36.8, 36.5,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com