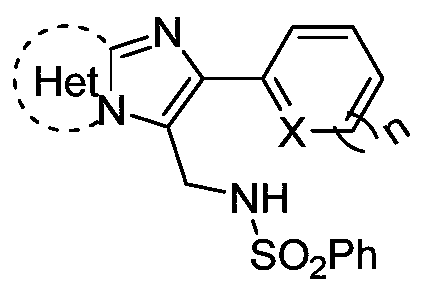
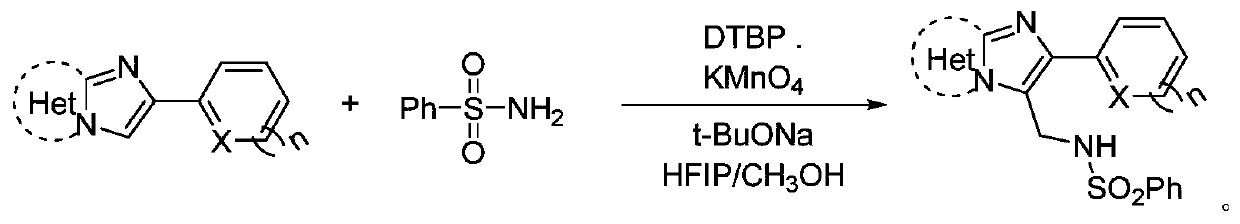
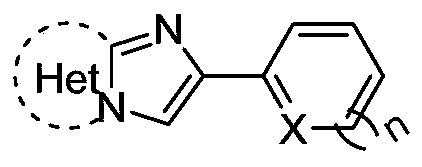
N-((2-heteroarylimidazoheteroaryl-3-yl)methyl)benzenesulfonamide compound and its synthesis method
A technology of heteroaryl imidazole and benzenesulfonamide, which is applied in the field of preparation of N-methyl)benzenesulfonamide compounds, can solve few problems and achieve the effect of wide applicability of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The structural formula of the compound N-((2-(thiophen-2-yl)imidazo[1,2-a]pyridin-3-yl)methyl)benzenesulfonamide of this embodiment is:
[0026]
[0027] The preparation method is: in an air environment, add 0.1mmol of 2-(thiophen-2-yl)imidazo[1,2-a]pyridine compound, 0.2mmol of benzenesulfonamide, 0.2mmol of di tert-butyl peroxide, 0.1 mmol of potassium permanganate, 0.1 mmol of sodium tert-butoxide, 2 mL of hexafluoroisopropanol and methanol (volume ratio 1:9), reacted at 130 ° C for 8 hours; after the reaction, chromatographic separation (silica gel 200-300 mesh, eluent: ethyl acetate / petroleum ether gradient elution, ratio from 0 / 100 to 100 / 0), and dried to obtain a light yellow solid with a yield of 73%. mp 222-223°C; 1 H NMR (600MHz, DMSO) δ8.36–8.17(m,2H),7.76(d,J=7.2Hz,2H),7.66–7.46(m,5H),7.31(s,2H),7.09(s, 1H), 6.97(t, J=5.8Hz, 1H), 4.56(d, J=3.4Hz, 2H); 13 C NMR (101MHz, DMSO) δ144.0, 139.7, 138.2, 137.0, 132.5, 129.0, 127.9, 126.5, 126.3, 125.5, 124.8, ...
Embodiment 2
[0029] The structural formula of the compound N-((2-(thiophen-2-yl)imidazo[1,2-a]pyridin-3-yl)methyl)benzenesulfonamide of this embodiment is:
[0030]
[0031] The preparation method is: in an air environment, add 0.1mmol of 2-(thiophen-2-yl)imidazo[1,2-a]pyridine compound, 0.3mmol of benzenesulfonamide, 0.1mmol of di tert-butyl peroxide, 0.2 mmol of potassium permanganate, 0.1 mmol of sodium tert-butoxide, 2 mL of hexafluoroisopropanol and methanol (volume ratio 1:9), reacted at 140 ° C for 10 hours; after the reaction, chromatographic separation (silica gel 200-300 mesh, eluent: gradient elution with ethyl acetate / petroleum ether, the ratio is from 0 / 100 to 100 / 0), and dried to obtain a light yellow solid with a yield of 74%. mp 222-223°C; 1 H NMR (600MHz, DMSO) δ8.36–8.17(m,2H),7.76(d,J=7.2Hz,2H),7.66–7.46(m,5H),7.31(s,2H),7.09(s, 1H), 6.97(t, J=5.8Hz, 1H), 4.56(d, J=3.4Hz, 2H); 13 C NMR (101MHz, DMSO) δ144.0, 139.7, 138.2, 137.0, 132.5, 129.0, 127.9, 126.5, 126.3, 1...
Embodiment 3
[0033] The structural formula of the compound N-((2-(furan-2-yl)imidazo[1,2-a]pyridin-3-yl)methyl)benzenesulfonamide of this embodiment is:
[0034]
[0035]The preparation method is: in an air environment, add 0.1mmol of 2-(furan-2-yl)imidazo[1,2-a]pyridine compound, 0.2mmol of benzenesulfonamide, 0.2mmol of tert-butyl peroxide, 0.1 mmol of potassium permanganate, 0.2 mmol of sodium tert-butoxide, 2 mL of hexafluoroisopropanol and methanol (volume ratio 1:9), reacted at 100 ° C for 12 hours; after the reaction, chromatographic separation (silica gel 200-300 mesh, eluent: ethyl acetate / petroleum ether gradient elution, ratio from 0 / 100 to 100 / 0), and dried to obtain a white solid with a yield of 48%. mp 236-237°C; 1 H NMR (600MHz, DMSO) δ8.31–8.16(m,2H),7.75(d,J=7.1Hz,2H),7.70–7.58(m,2H),7.54(t,J=8.4Hz,3H) ,7.32(t,J=7.3Hz,1H),6.98(s,1H),6.81(s,1H),6.59(s,1H),4.67(s,2H); 13 C NMR (101MHz, DMSO) δ149.4, 144.5, 142.9, 139.8, 135.0, 132.4, 129.0, 126.3, 125.5, 124.6, 116.5, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com