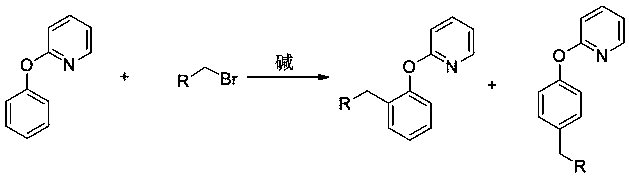
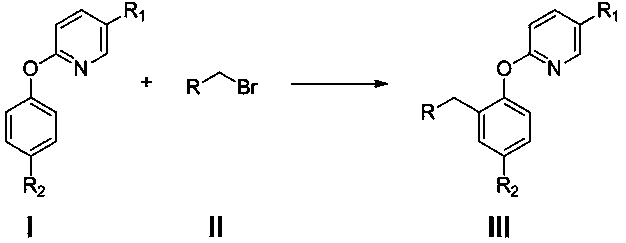
A kind of preparation method of 2-(2-alkylphenoxy)pyridine derivatives
A technology of alkylphenoxy and phenoxypyridine, applied in the field of chemistry, can solve the problems of difficult separation, high cost, difficult target product, etc., and achieve the effects of simple process, good specificity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
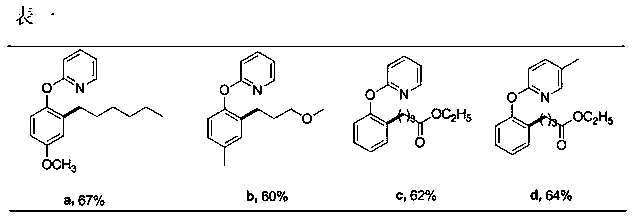
[0012] Add 40mg (0.2mmol) 2-(4-methoxyphenoxy)pyridine, 98mg (0.6mmol) 1-bromo-n-hexane, 6mg (0.01mmol) dichlorobis(4-methyl) into a 20mL pressure-resistant reaction tube Isopropylphenyl) ruthenium, 55mg (0.4mmol) potassium carbonate, 11mg (0.06mmol) 1-adamantanecarboxylic acid, 1.5mL benzene, sealed under nitrogen, heated to 120 ° C, stirred for 24 hours, after the reaction, After separation by column chromatography, 38 mg of the target product 2-(2-n-hexyl-4-methoxyphenoxy)pyridine was obtained with a yield of 67%.
Embodiment 2
[0014] Add 37mg (0.2mmol) 2-(4-methylphenoxy) pyridine, 92mg (0.6mmol) 1-bromo-3-methoxypropane, 6mg (0.01mmol) dichlorobis (4-Methylisopropylphenyl)ruthenium, 55mg (0.4mmol) potassium carbonate, 11mg (0.06mmol) 1-adamantanecarboxylic acid, 1.5mL benzene, sealed under nitrogen, heated to 120°C for reaction, stirred for 24 hours , after the reaction, separated by column chromatography to obtain 31 mg of the target product 2-(4-methyl-2-(3-methoxypropyl)phenoxy)pyridine with a yield of 60%.
Embodiment 3
[0016] Add 34mg (0.2mmol) 2-phenoxypyridine, 117mg (0.6 mmol) ethyl 4-bromobutyrate, 6mg (0.01mmol) dichlorobis(4-methylisopropylbenzene) into a 20mL pressure-resistant reaction tube base) ruthenium, 55mg (0.4mmol) potassium carbonate, 11mg (0.06mmol) 1-adamantanecarboxylic acid, 1.5mL benzene, sealed under nitrogen, heated to 120°C for reaction, stirred for 24 hours, after reaction, separated by column chromatography to obtain The target product 4-(2-(2-pyridyloxy)ethyl phenylbutyrate was 35 mg, and the yield was 62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com