Method for separation and purification of 2-adamantanecarboxylic acid and 1-adamantanecarboxylic acid
A technology of adamantanecarboxylic acid and a purification method is applied in the field of separation and purification of adamantanecarboxylic acid mixtures, and can solve problems such as difficulty in separation, difficulty in separation of mixtures, environmental pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
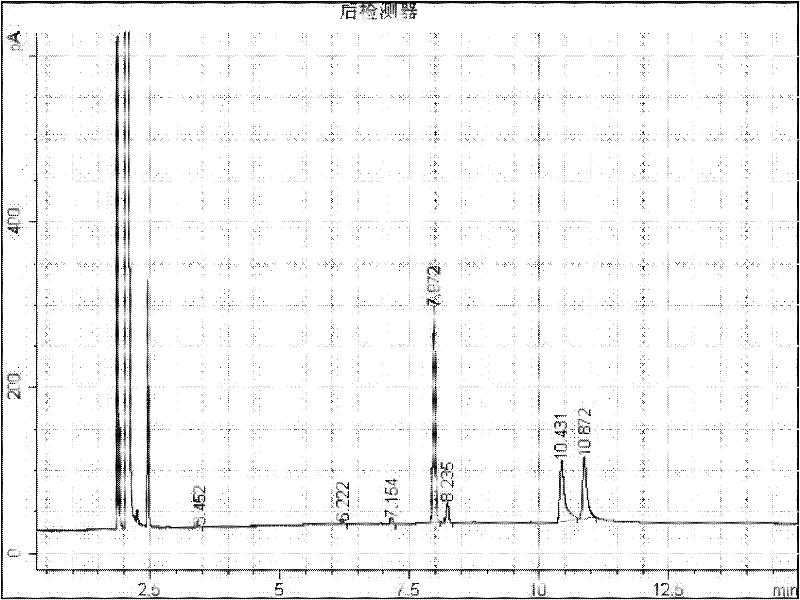
[0012] Add 2g of 1-adamantanecarboxylic acid and 2-adamantanecarboxylic acid mixture raw material (about 50:50) into a 50mL round bottom flask, add 10mL of methanol, add 0.2g of 98% sulfuric acid as a catalyst, heat up to 65°C with magnetic stirring carry out esterification reaction. After reacting for 15 minutes, the reaction system was sampled, 1 mL of water was added, extracted with 1 mL of ethyl acetate and injected into GC for analysis, the reaction results were as follows: figure 1 . where t R 7.972 is methyl 1-adamantanecarboxylate, t R 8.235 is methyl 2-adamantanecarboxylate, t R 10.431 is 1-adamantanecarboxylic acid, t R 10.872 is 2-adamantanecarboxylic acid. 33.5% of methyl 1-adamantanecarboxylate, 3.3% of methyl 2-adamantanecarboxylate, 16.9% of 1-adamantanecarboxylate, 41.3% of 2-adamantanecarboxylate. Stop the reaction for separation, add 20 mL of water, add 10% NaOH solution to alkaline (PH ≥ 10), separate the aqueous solution from the ester, acidify the aq...
Embodiment 2
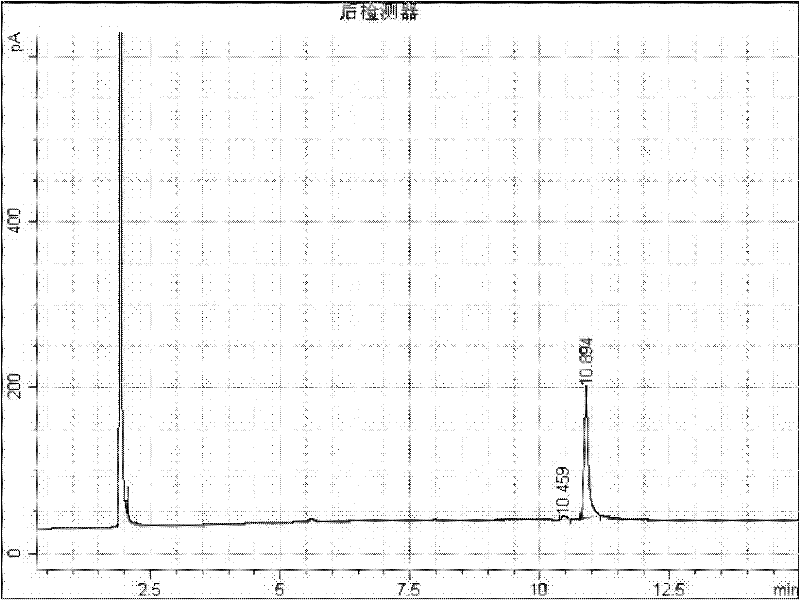
[0014] Add 2g of 1-adamantanecarboxylic acid and 2-adamantanecarboxylic acid mixture raw material (about 50:50) into a 50mL round bottom flask, add 10mL of methanol, add 0.2g of 98% sulfuric acid as a catalyst, heat up to 65°C with magnetic stirring carry out esterification reaction. After 30 minutes, it was detected that methyl 1-adamantanecarboxylate was 44.2%, methyl 2-adamantanecarboxylate was 4.3%, and 1-adamantanecarboxylate was 6.8%. 2-Adamantanecarboxylic acid 40.1%. Stop methylation reaction, adopt embodiment 1 identical aftertreatment, water layer obtains 0.6g of 2-adamantanecarboxylic acid, product fusing point 140-142 ℃, GC analysis content reaches 98.0%, as figure 2 . That 1 H NMR (400MH Z CDCl 3 )δ1.63-1.67(m 2H)δ1.68-1.78(m4H)δ1.86-1.93(m 6H)δ2.35(s 2H)δ2.67(s 1H)δ12.4(s 1H); 13 C NMR (100MH Z CDCl 3 ) 27.36 27.37 29.37 33.54 37.31 38.06 49.44 181.21. 0.8 g of 1-adamantanemethanol was obtained from the ester layer, and the content of GC analysis reach...
Embodiment 3
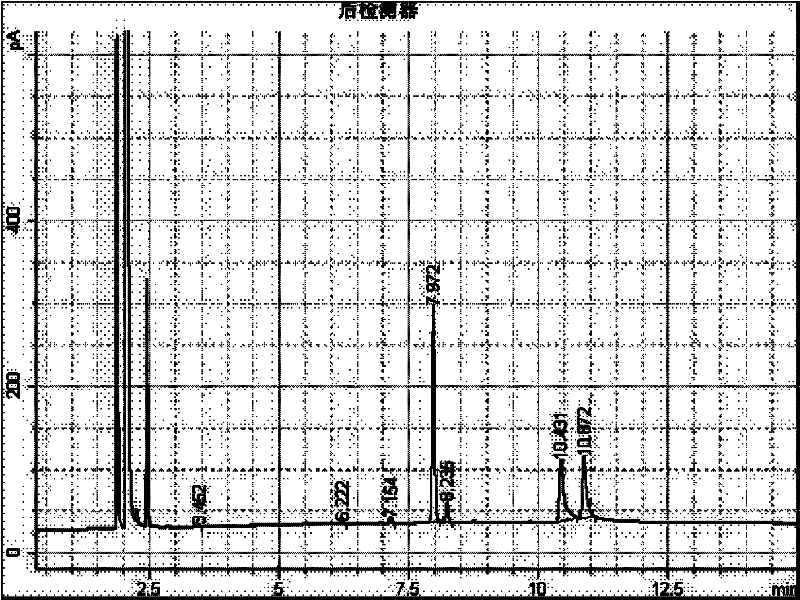
[0016] Add 2g of 1-adamantanecarboxylic acid and 2-adamantanecarboxylic acid mixture raw material (about 50:50) into a 50mL round bottom flask, add 10mL of methanol, dropwise add 0.8g of 98% sulfuric acid as a catalyst, and heat up to 65°C. Stir to carry out the esterification reaction. After reacting for 15 minutes, GC analysis showed that methyl 1-adamantanecarboxylate was 51.6%, methyl 2-adamantanecarboxylate was 41.2%, and 2-adamantanecarboxylate was 6.3%. 1-Adamantanecarboxylic acid 0.8%. After reacting for 30 minutes, methyl 1-adamantanecarboxylate was 52.3%, methyl 2-adamantanecarboxylate was 43.0%, and 2-adamantanecarboxylate was 4.5%. 1-Adamantanecarboxylic acid 0.3%. Less than 0.1 g of 2-adamantanecarboxylic acid was isolated. It shows that the amount of sulfuric acid catalyst is increased, and the rate of methylation is accelerated. If the reaction speed is too fast, it will be difficult to control the end point of the reaction. If the methyl esterification rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com