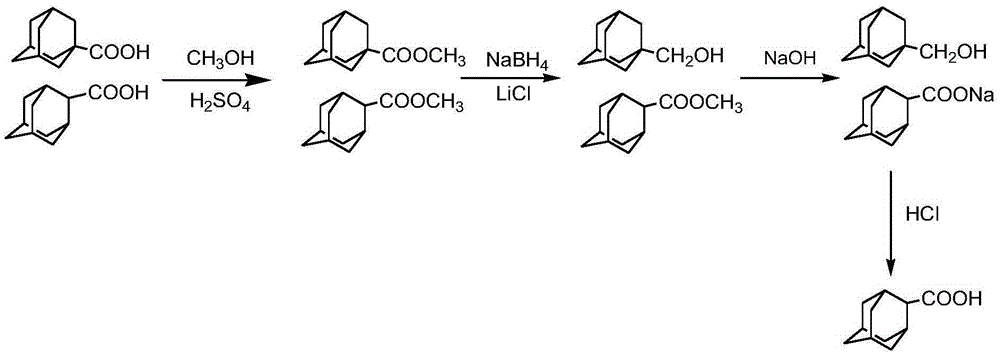
2-adamantanecarboxylic acid and 1-adamantane methanol separation and purification method
A technology of adamantane carboxylic acid and adamantane methanol, applied in the separation/purification of carboxylic acid compounds, separation/purification of hydroxyl compounds, chemical instruments and methods, etc., can solve the problems of difficult separation of mixtures, difficult separation, and environmental pollution. Achieve the effects of improving product purity, thorough esterification, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
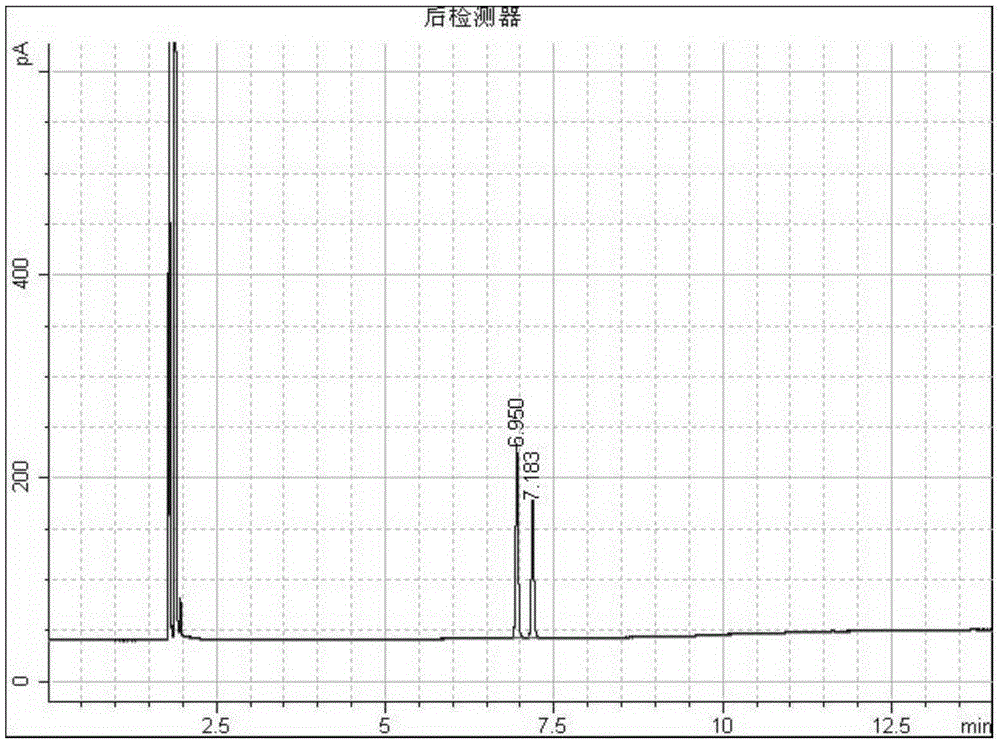
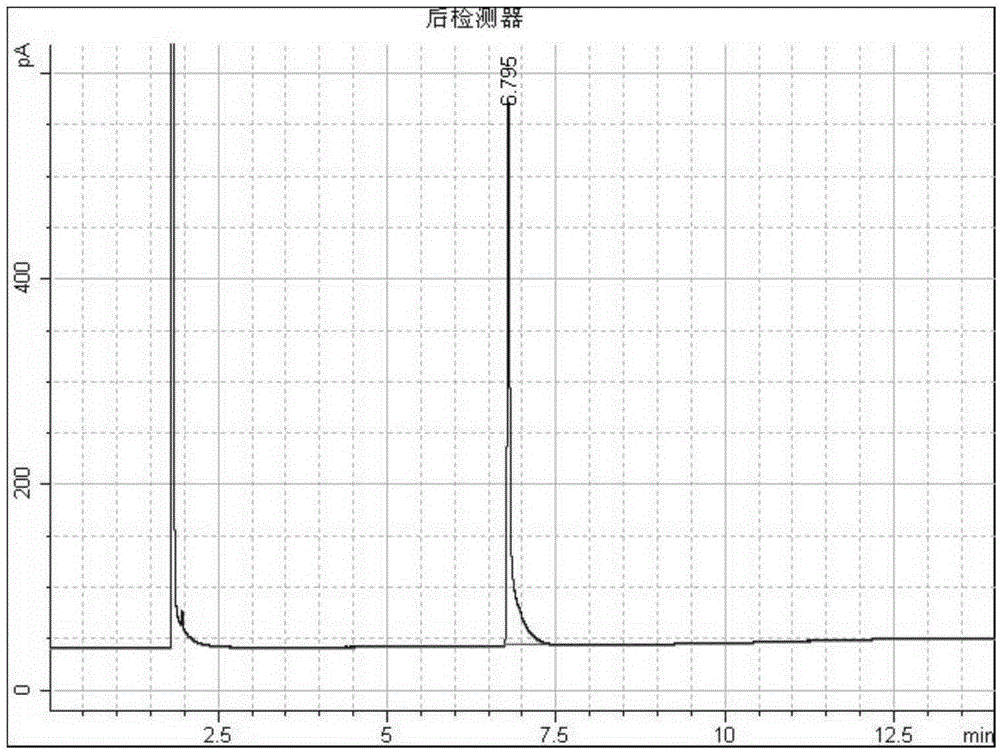
[0030] Such as figure 1 As shown, add 10 grams of 1-adamantanecarboxylic acid and 2-adamantanecarboxylic acid mixture raw materials (mass ratio 1:1) into a 100mL round bottom flask, add 30mL of methanol, add 3 grams of mass fraction 98% sulfuric acid as a catalyst, Raise the temperature to 65°C and carry out the esterification reaction with magnetic stirring. After reacting for 30 minutes, the reaction system was sampled, 1 mL of water was added, extracted with 1 mL of ethyl acetate and injected into GC for analysis. GC analysis results such as figure 2 Shown, wherein tR6.950 is 1-adamantane carboxylate, tR7.183 is 2-adamantane carboxylate. Stop the reaction for separation, add 60 mL of ice water, and separate the liquids. 10.8 g of the separated mixed methyl adamantane carboxylate was added, 22 mL of isopropanol was added, the water bath was refluxed for reaction, and 1.6 g of sodium borohydride and 1.8 g of anhydrous lithium chloride were added in batches to reduce the r...
Embodiment 2
[0032]In the 100mL round bottom flask that 10 grams of 1-adamantanecarboxylic acid and 2-adamantanecarboxylic acid mixture raw material (1-adamantanecarboxylic acid about 95%, 2-adamantanecarboxylic acid about 5%) were added, 30mL of methanol was added, and 4 Sulfuric acid with a gram mass fraction of 98% was used as a catalyst, and the temperature was raised to 55°C with magnetic stirring to carry out the esterification reaction. After reacting for 60 minutes, stop the esterification reaction for separation, add 60 mL of ice water, and separate by liquid separation. Add 55 mL of isopropanol to the separated methyl adamantane carboxylate, reflux in a water bath, add 2.6 g of sodium borohydride and 2.9 g of anhydrous lithium chloride in batches, and reduce the reaction for about 30 minutes to complete. Add 100 mL of ice water, separate the layers, add 2 mL of 10% NaOH solution to the organic layer, reflux for hydrolysis for 30 min, and saponify completely. Add 50 mL of ice wat...
Embodiment 3
[0034] Add 10g of 1-adamantanecarboxylic acid and 2-adamantanecarboxylic acid mixture raw material (about 25% of 1-adamantanecarboxylic acid, about 75% of 2-adamantanecarboxylic acid) into a 100mL round bottom flask, add 20mL of methanol, add 2 grams of mass Sulfuric acid with a fraction of 98% was used as a catalyst, and the temperature was raised to 70° C. in a water bath, refluxed, and magnetically stirred to carry out esterification. After reacting for 30 minutes, stop the esterification reaction for separation, add 40 mL of ice water, and separate by liquid separation. Add 55 mL of isopropanol to the isolated mixed methyl adamantane carboxylate, reflux in a water bath, add 1.4 g of sodium borohydride and 1.5 g of anhydrous lithium chloride in batches, and reduce the reaction for about 30 minutes to complete. Add 100 mL of ice water, separate the layers, and add 30 mL of 10% NaOH solution to the organic layer for reflux hydrolysis for 60 minutes to complete saponification....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com