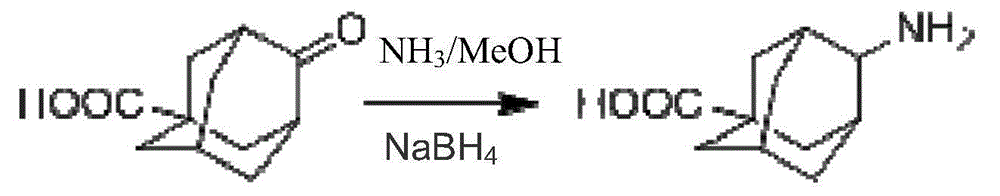Method for preparing 4-amino-adamantanecarboxylic acid
A technology of adamantanecarboxylic acid and amino group is applied in the preparation of organic compounds, chemical instruments and methods, cyanide reaction preparation and other directions, which can solve the problems of complex preparation method and high cost, and achieve cost reduction, good economic benefits, and operability. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
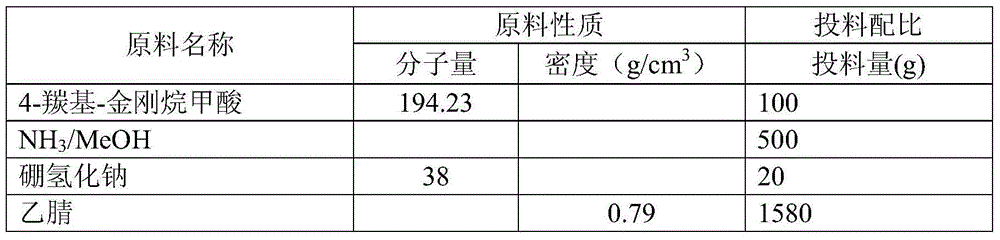
[0017] In a 2L reaction flask, add 100g of 4-carbonyl-adamantanecarboxylic acid and 500g of 7N NH 3 / MeOH solution, stirred at room temperature for 16 hours, cooled the reaction solution to 0°C, added 20g of sodium borohydride, stirred at 0-20°C for 4 hours, spin-dried the reaction solution, added 2L of water and stirred for 30 minutes, adjusted the pH to 5 with hydrochloric acid -6, continue to stir for 30 minutes, extract the reaction solution with 300mL dichloromethane for 3 times, combine the dichloromethane, spin dry, add 1L acetonitrile and 1L water to the solid, stir at room temperature for 10 hours, and obtain the solid product after suction filtration and drying. 90 g of product.
[0018] The NMR data are as follows:
[0019] 1H NMR (400MHz, CD3OD-d4) δ 3.36 (m, 1H), 2.03-1.53 (m, 13H).
[0020] The material ratio is as follows:
[0021]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com