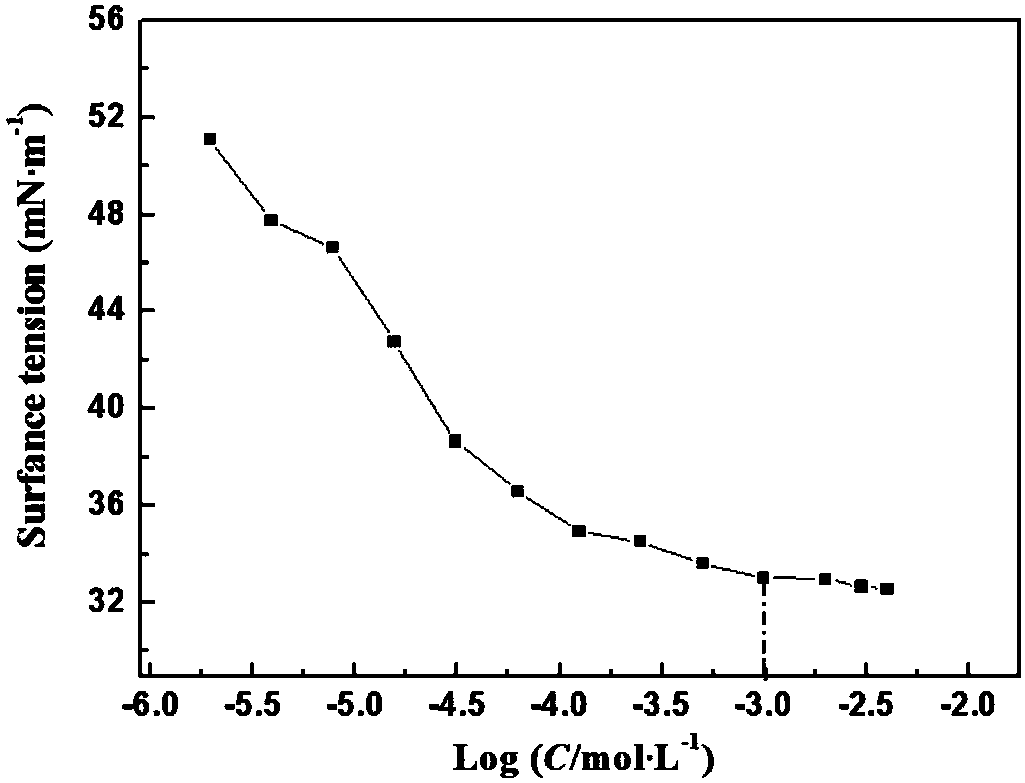
Adamantane-containing Gemini surfactant and synthesis method thereof
A technique for surfactants and synthesis methods, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of Gemini surfactants that have not been reported in literature, and achieve improved amphiphilicity and easy availability of raw materials , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
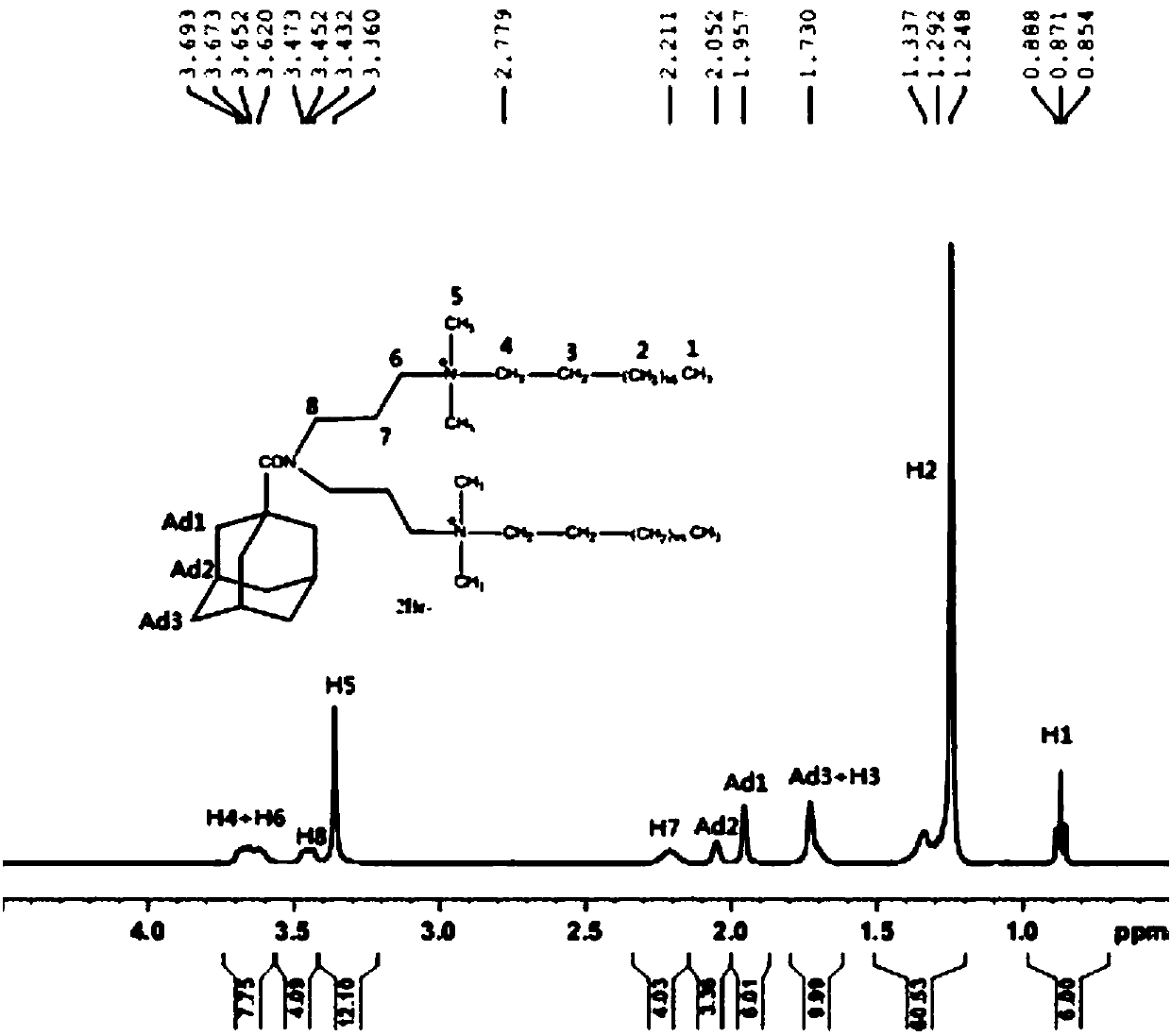
[0024] Embodiment 1: Gemini surfactant containing adamantane (R=C 12 h 25 )Synthesis
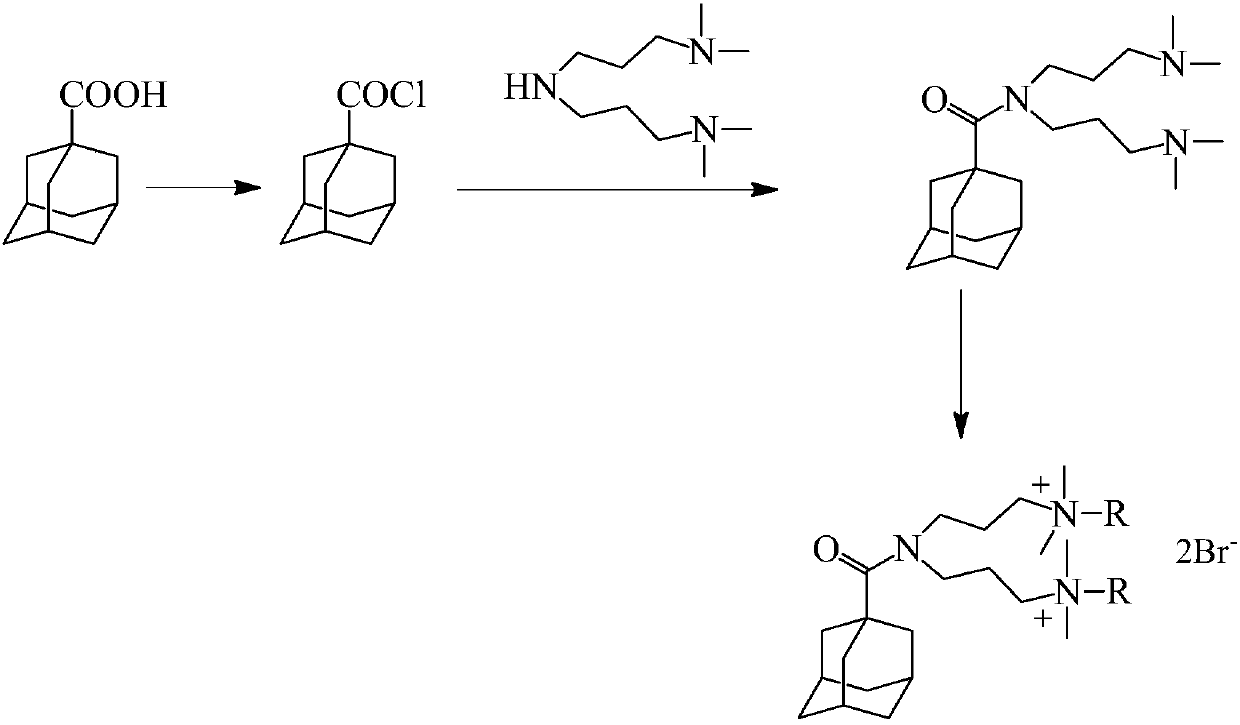
[0025] Weigh 1.8g (0.01mol) of 1-adamantanecarboxylic acid into a 100mL three-necked flask (installed with a thermometer, constant pressure dropping funnel, condenser tube, magnetic stirring), and drop 2.4g of thionyl chloride into the bottle to form a suspension liquid, heated up to 80°C under the protection of nitrogen to react until the solution was a transparent clear liquid, and evaporated to dryness under reduced pressure after completion to obtain yellow 1-adamantanecarbonyl chloride solid with a yield of 95%.
[0026] Under the protection of nitrogen, dissolve 2.0g (0.01mol) of 1-adamantanecarbonyl chloride in ether, keep the temperature at 0°C, and add 2.4g (0.013mol) of 3,3'-iminobis(N, The solution formed by N-dimethylpropylamine) and diethyl ether has a white precipitate. After the dropwise addition, the temperature is raised to 40°C for 5 hours. The precipitate is filtered to ...
Embodiment 2
[0028] Embodiment 2: Gemini surfactant containing adamantane (R=C 16 h 33 )Synthesis
[0029] Weigh 1.8g (0.01mol) of 1-adamantanecarboxylic acid and add it to a 100mL three-neck flask (installed with a thermometer, constant pressure dropping funnel, condenser tube, magnetic stirring), and drop 6.0g of thionyl chloride into the bottle to form a suspension , N 2 In the atmosphere, the temperature was raised to 60° C. to react until the solution was a transparent clear liquid, and then evaporated to dryness under reduced pressure to obtain a yellow 1-adamantanecarbonyl chloride solid with a yield of 97%.
[0030] Under the protection of nitrogen, dissolve 2.0g (0.01mol) of 1-adamantanecarbonyl chloride in ether, keep the temperature at 20°C, and add 2.2g (0.012mol) of 3,3'-iminobis(N, The solution formed by N-dimethylpropylamine) and diethyl ether has a white precipitate. After the dropwise addition, the temperature is raised to 30°C for 10 hours. The precipitate is filtered ...
Embodiment 3
[0032] Embodiment 3: Gemini surfactant containing adamantane (R=C 18 h 37 )Synthesis
[0033] Weigh 1.8g (0.01mol) of 1-adamantanecarboxylic acid and add it to a 100mL three-necked flask (installed with a thermometer, constant pressure dropping funnel, condenser tube, magnetic stirring), and drop 4.8g of thionyl chloride into the bottle to form a suspension , N 2 In the atmosphere, the temperature was raised to 70° C. to react until the solution was a transparent clear liquid. After completion, it was evaporated to dryness under reduced pressure to obtain a yellow 1-adamantanecarbonyl chloride solid with a yield of 96%.
[0034] Under the protection of nitrogen, dissolve 2.0g (0.01mol) of 1-adamantanecarbonyl chloride in diethyl ether, keep the temperature at 15°C, and add 2.1g (0.011mol) of 3,3'-iminobis(N, The solution formed by N-dimethylpropylamine) and diethyl ether produced white precipitates. After the dropwise addition, the temperature was raised to 25°C for 8 hours, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com