A kind of amphiphilic fluorescent conjugated polymer, preparation method and application
A conjugated polymer and amphiphilic technology, applied in the field of fluorescent sensing materials, to achieve good selectivity and high sensitivity, enhanced amphiphilicity, and strong interaction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
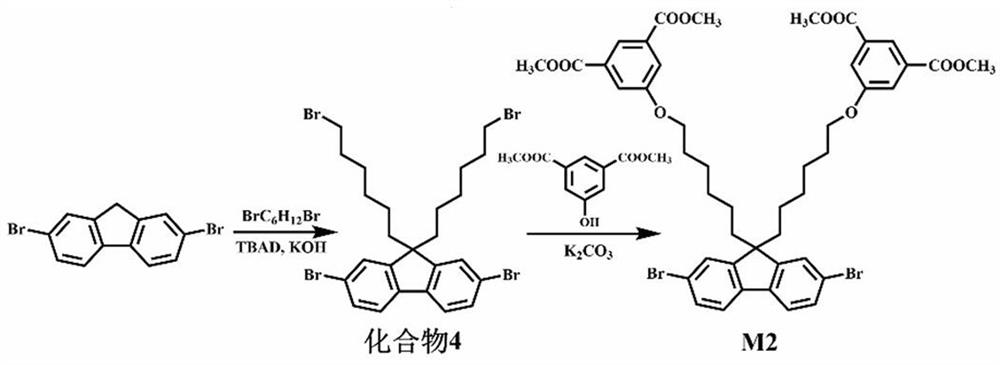
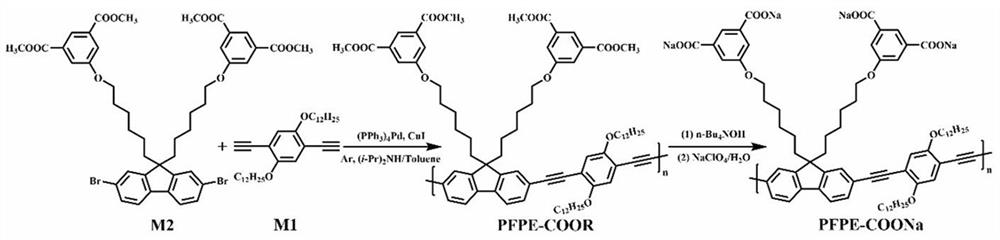
[0049] 1. Synthesis of Monomers M1 and M2
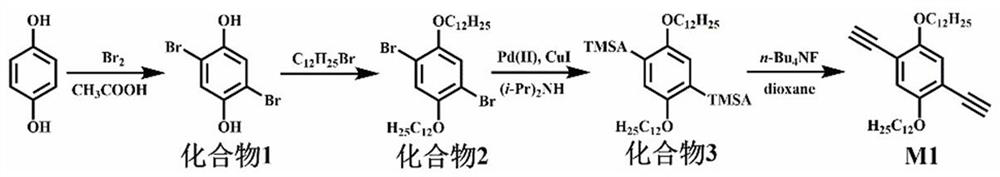
[0050] (1) See attached figure 1 , which is a synthetic route diagram of the monomer M1 required for the preparation of fluorescent conjugated polymers. In this example, the monomer M1 is 1,4-diethynyl-2,5-didodecyloxybenzene.
[0051] figure 1 , the preparation of compound 1 (2,5-dibromohydroquinone): Weigh a certain amount of hydroquinone (16.201 g, 147 mmol) in a 500 mL double-necked flask, add acetic acid (82 mL) dissolve. Then measure 68 mL of acetic acid into the constant pressure dropping funnel, use a disposable medical syringe to draw 16 mL of liquid bromine and mix it with the acetic acid in the constant pressure dropping funnel. During the reaction, an ice-water bath was used, and the mixed solution of liquid bromine and acetic acid was slowly added dropwise, and the reaction was carried out overnight. After the reaction, the mixture in the flask was vacuum-filtered, and the solid product was recrystallized using met...
Embodiment 2
[0068] In this example, the photophysical properties of the polymer PFPE-COONa synthesized in Example 1 were measured. See attached Figure 9 , which is polymer PFPE-COONa in different THF / H 2 Fluorescence emission spectra in mixed solvents with O ratio. Depend on Figure 9 It can be seen that the polymer PFPE-COONa in different THF / H 2 The mixed solvent with O ratio has special photophysical properties and can be used in sensing and detection.
Embodiment 3
[0070] In this example, the polymer-conjugated polymer PFPE-COONa provided in Example 1 was used to detect neomycin.
[0071] Experimental method: Dissolving the polymer PFPE-COONa in THF / H 2 In O=1:1 mixed solvent, the concentration is 5×10-6 M (according to repeat unit), stir for 24 hours until the solution is stable; then, take 20 mL of the mixed solution in a 50 mL Erlenmeyer flask for detection experiments; configuration of the analyte solution: Neomycin is configured to a concentration of 1×10 -3 mol / L deionized aqueous solution; add a very small amount of neomycin solution (2 μL) into the Erlenmeyer flask each time, and calculate the concentration of neomycin in the solution to be tested; in actual detection, the set analyte The concentration gradient is 0, 0.1, 0.2, 0.3...1.6, 1.7 and 1.8 μM. Through this preparation method, the experimental error caused by the volume change of the solvent during the titration process can be ignored (the error range is less than 1%). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com