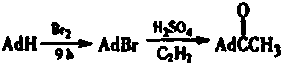Preparation method of 1-adamantyl methy ketone
A technology of adamantane methyl ketone and adamantane carboxylic acid, which is applied in the field of preparation of 1-adamantane methyl ketone, can solve the problems of low yield, achieve cost reduction, facilitate post-processing, and protect the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A preparation method for 1-adamantyl methyl ketone, comprising the steps of:
[0020] Step 1: In the reaction flask, add 2.5mol adamantane and 4.5mol liquid bromine in sequence, heat up at 80-90°C for 6h, then react at 110-115°C (oil temperature) for 3h, and let stand overnight. Recover 30 mL of bromine by distillation, then reduce the remaining bromine with saturated sodium bisulfite solution (200 mL), filter, wash the filter cake with water until neutral, dry, and recrystallize from methanol to obtain 470 g of light yellow crystalline 1-bromoadamantane, yield : 92.7%, mp117-119℃;
[0021] Step 2: In a 1000mL flask, add 98.4% concentrated sulfuric acid (500mL), start stirring, at about 5-10°C, add 0.1mol 1-bromoadamantane, 50mL n-hexane, stir rapidly, add dropwise 45mL formic acid, After 1 hour of dripping, keep the temperature at 5-10°C and react for 3 hours. Pour the reaction solution into ice water to obtain a mixture of ice and water (1500 mL), let it stand, filt...
Embodiment 2
[0026] A preparation method for 1-adamantyl methyl ketone, comprising the steps of:
[0027] Step 1: Add 2.5 mol of adamantane and 4.9 mol of liquid bromine in sequence in the reaction flask, heat up at 80-90°C for 6 hours, then react at 110-115°C (oil temperature) for 3 hours, and let stand overnight. Recover 30 mL of bromine by distillation, then reduce the remaining bromine with saturated sodium bisulfite solution (200 mL), filter, wash the filter cake with water until neutral, dry, and recrystallize from methanol to obtain 470 g of light yellow crystalline 1-bromoadamantane, yield : 92.4%, mp117-119℃;
[0028] Step 2: In a 1000mL flask, add 98.4% concentrated sulfuric acid (500mL), start stirring, at about 5-10°C, add 0.1mol 1-bromoadamantane, 55mL n-hexane, stir rapidly, add 45mL formic acid dropwise, After 1 hour of dripping, keep the temperature at 5-10°C and react for 3 hours. Pour the reaction solution into ice water to obtain a mixture of ice and water (1500 mL), l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com