Method for synthesizing 2-(1-imidazol)ethylamine
A synthesis method and imidazole-based technology are applied in the field of preparation of fine chemicals, can solve the problems of expensive 2-ethyl-2-oxazoline and difficult to obtain propionamide, and achieve high total yield, mild conditions and convenience Effects of manipulation and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
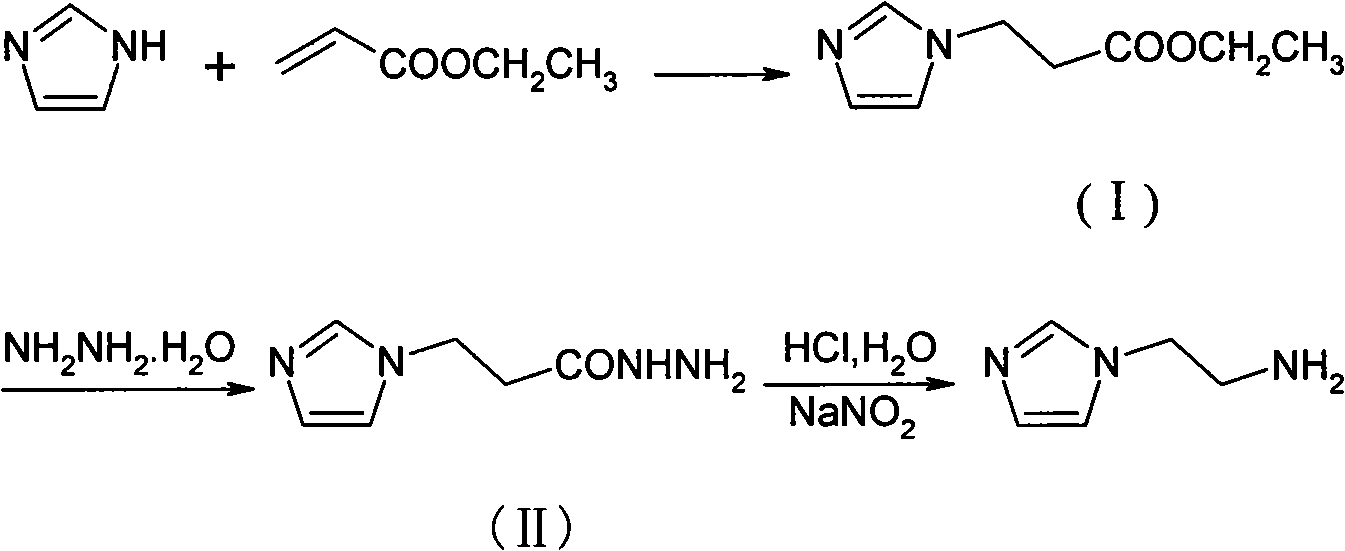
[0017] (1), the preparation of intermediate (I): add imidazole (4.1g, 60mmol), TEA (1.0ml, 7.2mmol), methanol (60ml) successively in 150ml there-necked flask, slowly add ethyl acrylate (7.7ml , 72mmol), stirred, reacted at 50°C for 5h, column chromatography (CH 2 Cl 2 :CH 3 OH=40:1) to isolate a pale yellow liquid compound (9.4 g) with a yield of 94.8%. 1 HNMR (Cl 3 CD-d 6 )δ: 7.50(s, 1H), 7.04(s, 1H), 6.94(s, 1H), 4.26(t, 2H), 4.13(q, 2H), 2.77(t, 2H), 1.24(t, 3H ).
[0018] (2), the preparation of intermediate (II): 80% hydrazine hydrate (7.5ml, 150mmol), ethanol (30ml), slowly drop the mixture of ethanol (10ml) and intermediate (I) (8.40g, 50mmol), The temperature was not higher than 40°C. After stirring for about 30 minutes, the temperature was raised to reflux for 8 hours. Column chromatography (CH 2 Cl 2 :CH 3 OH=20:3) to isolate yellow compound 2 (7.26g), yield 94.3%. 1 HNMR (Cl 3 CD-d 6 )δ: 8.41(s, 1H), 7.52(s, 1H), 7.04(s, 1H), 6.91(s, 1H), 4.32(t, 2H), 3....
example 2
[0021] Intermediate (I) was prepared according to the method of Example 1, using acetonitrile as solvent, and the rest of the operations were the same.
example 3
[0023] Intermediate (I) was prepared according to the method of Example 1, THF was used as solvent, and the rest of the operations were the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com