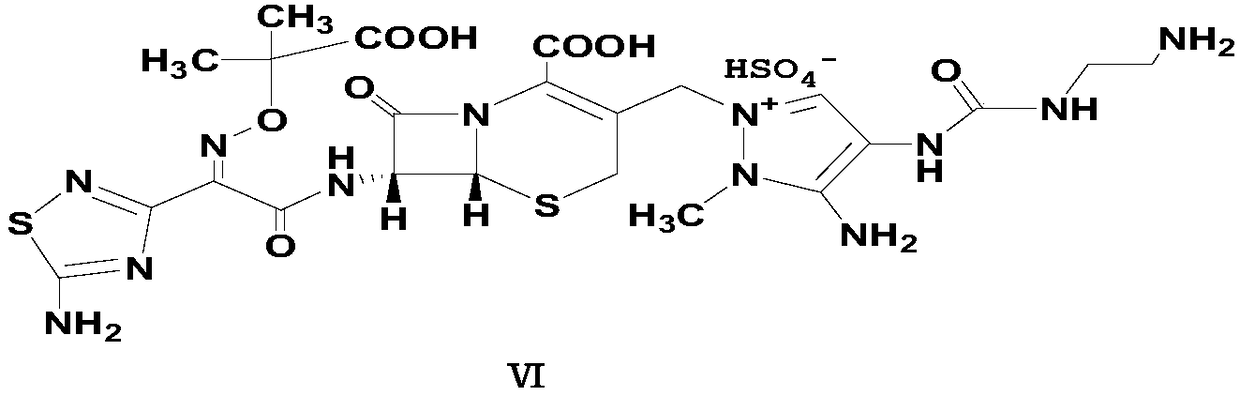
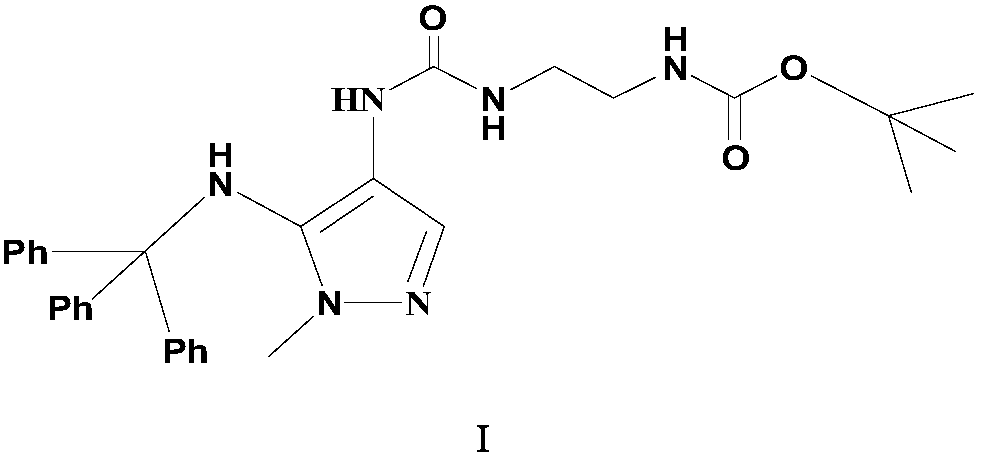
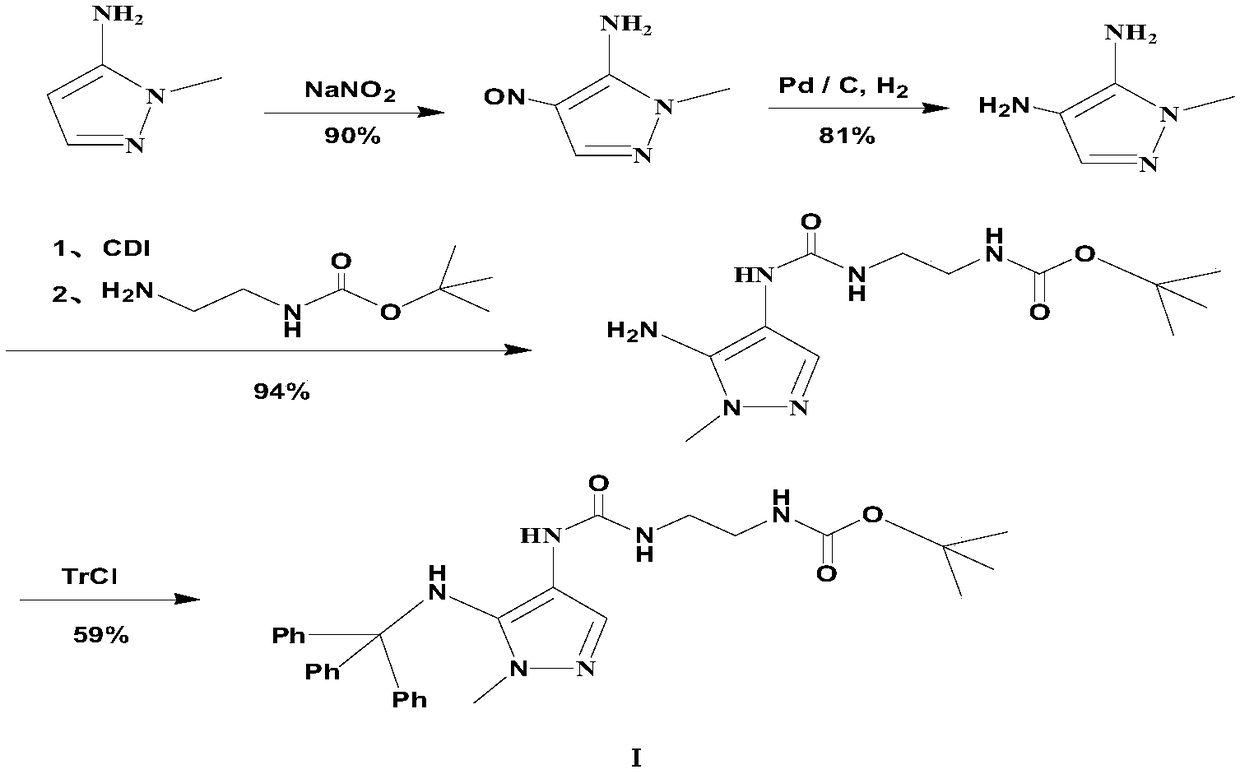
Preparation method of carbamyl amino pyrazol derived compound
A carbamoylaminopyrazole and compound technology, which is applied in the field of preparation of carbamoylaminopyrazole derivative compounds, can solve the problems of high price and low total yield, reduce material loss, improve yield and purity , The effect of simplifying the process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Dissolve 2 g of the starting material 1-methyl-5-amino-4-ethoxycarbonylpyrazole in 24 mL of acetone at room temperature, and stir to dissolve. Then add 4.9g of triphenylchloromethane, stir and dissolve, then add 1.2g of pyridine dropwise, after the dropwise addition, heat up to 35°C gradually, and carry out the condensation reaction with heat preservation and stirring for 2h. During the reaction, solids are precipitated. , the temperature of the reaction solution was gradually lowered to about 5°C, and after stirring for 1 h, the reaction solution was filtered to remove salt components, and the filter cake was washed with 2 mL of acetone to finally obtain an acetone solution containing the corresponding intermediate compound of formula III for use.
[0051] At room temperature, put 3.84g of sodium hydroxide into another clean three-necked reaction flask, then slowly add 5mL of water and stir to dissolve the alkali completely. After the temperature of the aqueous solution...
Embodiment 2
[0054] Dissolve 2 g of the starting material 1-methyl-5-amino-4-ethoxycarbonylpyrazole in 30 mL of tetrahydrofuran at room temperature, and stir to dissolve. Then add 5.2 g of triphenylbromethane, stir to dissolve, then add 1.5 g of triethylamine dropwise, after the dropwise addition, heat up to 40°C and carry out the condensation reaction with heat preservation and stirring for 2.5 hours. During the reaction, solids are precipitated. After the end, the temperature of the reaction solution was gradually lowered to about 0°C, and after stirring for 1 h, the reaction solution was filtered to remove salt components, and then the filter cake was washed with 5 mL of tetrahydrofuran to finally obtain a tetrahydrofuran solution containing the corresponding intermediate compound of formula III. use.
[0055]At room temperature, put 4.0g of potassium hydroxide into another clean three-necked reaction flask, then slowly add 10mL of water and stir to dissolve the alkali completely. After...
Embodiment 3
[0058] The specific operation before the corresponding step C in this embodiment is the same as that of the embodiment 1, and will not be repeated here. Specifically, the compound of the intermediate product formula IV before the step C is obtained by using the implementation method of the corresponding operation in the embodiment 1, and then, The specific implementation of the next step is as follows:
[0059] Then take 3.8g (about 0.01mol) of the solid compound of formula IV and put it into another clean three-necked reaction flask, then add 60mL of dichloromethane, stir to dissolve, then add 3.0g of triethylamine, stir and mix evenly, and control the temperature at 20°C~25°C, dropwise add 2.0g (about 0.03mol) of sodium azide into the reaction liquid, after the dropwise addition, gradually raise the temperature of the system to 50°C, and carry out the rearrangement reaction with stirring and heat preservation for 3.0h, the reaction process is Gas generation, pay attention to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com