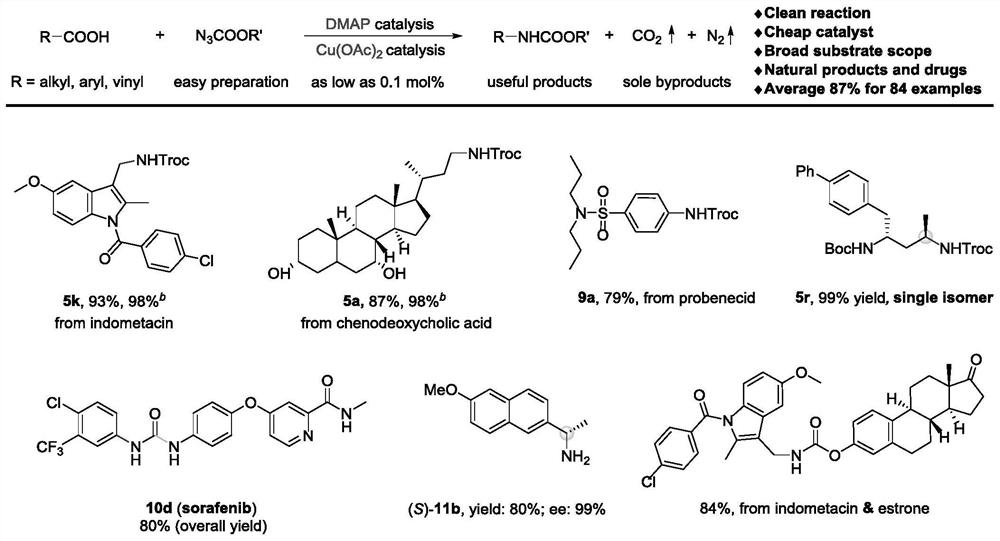
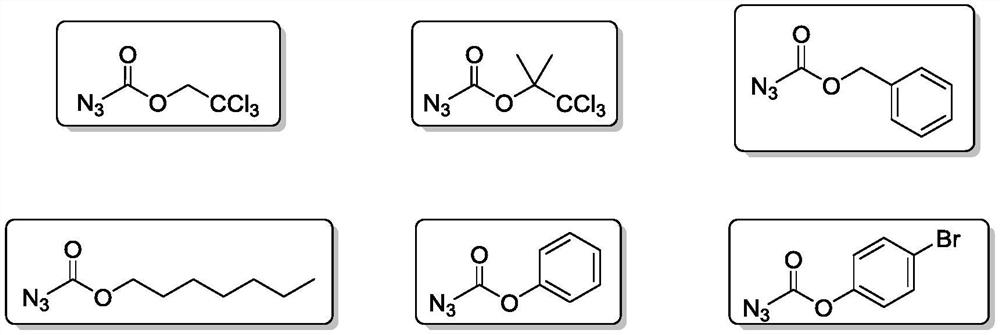
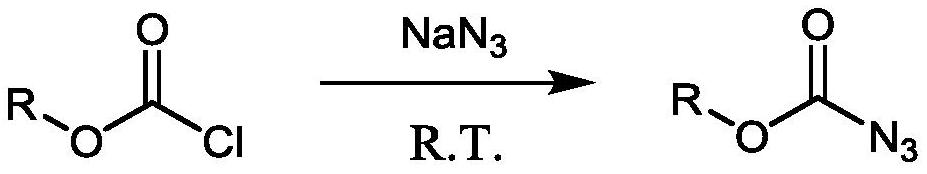Method for preparing amine compounds based on novel catalytic curtius rearrangement reaction
A technology of amine compounds and rearrangement reactions, which is applied in the preparation of organic compounds, preparation of amino hydroxyl compounds, steroids, etc., can solve problems such as few C-N bonds, and achieve high atom economy, huge development potential, and operation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Preparation of 2,2,2-trichloroethyl-((1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-)methyl)carbamate ester
[0054] The target product passes through the reaction process and post-processing. Purification by column chromatography afforded 94.0 mg of the target product (93% isolated yield).
[0055] 1 H NMR (400MHz, Chloroform-d) δ7.64(d, J=8.5Hz, 2H), 7.49(d, J=8.4Hz, 2H), 7.03(d, J=2.5Hz, 1H), 6.81(d ,J=9.0Hz,1H),6.66(dd,J=9.1,2.6Hz,1H),5.25(t,J=5.5Hz,1H),4.76(s,2H),4.53(d,J=5.5Hz ,2H),3.81(s,3H),2.42(s,3H); 13 C NMR (101MHz, Chloroform-d) δ168.4, 156.2, 154.7, 139.6, 136.5, 133.7, 131.3, 131.0, 129.9, 129.3, 115.6, 115.1, 112.1, 101.3, 95.7, 74.7, 55.8, 35.4, 13.2; )v 3344, 2929, 1732, 1682, 1591, 1478, 1221, 1045, 811, 721cm -1 ; HRMS (ESI) Calcd.for C21 h 18 Cl 4 N 2 o 4 Na[M+Na] + 524.9913,found 524.9908.
Embodiment 2
[0057] Preparation of 2,2,2-trichloroethyl((R)-3-((3R,7R,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethyl Hexadecylhydro-1H-cyclopenta[a]phenanthrene-17-)butyl)carbamate
[0058]
[0059] The target product passes through the reaction process and post-processing. Purification by column chromatography afforded 93.2 mg of the target product (87% isolated yield).
[0060] 1 H NMR (400MHz, Chloroform-d) δ4.95(t, J=6.1Hz, 1H), 4.72(s, 2H), 3.84(q, J=3.0Hz, 1H), 3.49–3.42(m, 1H) ,3.34–3.26(m,1H),3.21–3.12(m,1H),2.19(q,J=12.7Hz,1H),2.00–1.94(m,2H),1.92–1.86(m,1H),1.84 –1.78(m,2H),1.72–1.59(m,4H),1.52–1.45(m,6H),1.41–1.33(m,3H),1.31–1.09(m,7H),0.97(d,J= 6.6Hz, 4H), 0.90(s, 3H), 0.65(s, 3H); 13 C NMR (101MHz, Chloroform-d) δ154.6, 95.9, 74.6, 72.1, 68.6, 56.1, 50.6, 42.9, 41.6, 40.0, 39.7, 39.5, 39.0, 36.0, 35.4, 35.2, 34.8, 33.9, 32.9, 30.8 ,28.5,23.8,22.9,20.7,18.7,11.9; IR (neat) v3445,3347,2932,2867,2133,1715,1520,1251,1141,731cm -1 ; HRMS (ESI) Calcd.for C 26 h 42 C...
Embodiment 3
[0062] Preparation of 2,2,2-trichloroethyl(4-(N,N-dipropylsulfamoyl)phenyl)carbamate
[0063]
[0064] The target product passes through the reaction process and post-processing. Purification by column chromatography afforded 67.8 mg of the target product (79% isolated yield). 1 H NMR (400MHz, Chloroform-d) δ7.77 (d, J = 8.7Hz, 2H), 7.57 (d, J = 8.7Hz, 2H), 7.29 (brs, 1H), 4.84 (s, 2H), 3.08 –3.04(m,4H),1.59–1.50(m,4H),0.86(t,J=7.4Hz,6H); 13 C NMR (101MHz, Chloroform-d) δ151.4, 140.9, 135.3, 128.6, 118.6, 95.1, 74.8, 50.2, 22.1, 11.3; IR (neat) v3319, 2966, 1751, 1596, 1533, 1207, 1151, 590cm -1 ; HRMS (ESI) Calcd.for C 15 h 22 Cl 3 N 2 o 4 S[M+H] + 431.0361,found 431.0362.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com