Preparation method of trans-N-Boc-1,3-cyclobutanediamine
A technology of n-boc-1 and cyclobutylene diamine, which is applied to the preparation of organic compounds, chemical instruments and methods, and the preparation of carbamic acid derivatives. It can solve the problems of long steps and low yields, and achieve short steps , simple operation, simple and easy purification method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] step one:
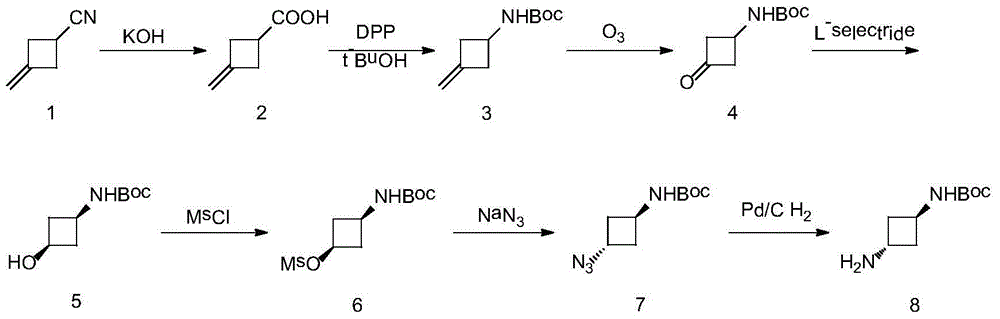
[0032] To a solution of 3-methylenecyclobutanecarbonitrile (400 g, 4.3 mol) in ethanol (5 L) and water (5 L) was added potassium hydroxide (2408 g, 43 mol). After refluxing for 2 hours, the pH of the solution was adjusted to 2 with concentrated hydrochloric acid. The precipitated white solid was filtered, washed with water, and dried to obtain Compound 2 (450 g), with a yield of 93.4%. Proton NMR spectrum (400MHz, CDCl 3 )δ: 2.67-3.56 (5H, m), 4.68-4.98 (2H, m), 10.73 (1H, br, s), indicating that the target product was obtained. Its molecular weight is 112.13.
[0033] Step two:
[0034] Triethylamine (590g, 5.9mol), diphenylphosphoryl azide (DPPA) (1273g, 4.7mol) and compound 2 (440g, 3.9mol) were dissolved in tert-butanol (8L) at a reflux temperature of 83°C in sequence , and then reflux for 12 hours. The reaction was quenched with water (2 L), and after the solution was concentrated to 1 / 5 of the original volume, ethyl acetate (7.8 L) was added to t...
Embodiment 2
[0046] step one:
[0047] Same as Step 1 in Example 1.
[0048] Step two:
[0049] Potassium carbonate (814g, 5.9mol), NaN 3 (305.5g, 4.7mol) and compound 2 (440g, 3.9mol) were dissolved in tert-butanol (8L) at a reflux temperature of 83°C, and then refluxed for 12 hours. The reaction was quenched with water (2 L), and after the solvent was concentrated, ethyl acetate (7.8 L) was added to the remaining solution. The organic phase was washed with water, washed with saturated brine, dried, and spin-dried to obtain a white solid, compound 3 (520.6 g), with a yield of 73%. Proton NMR spectrum (400MHz, CDCl 3 )δ:1.36(9H,s),2.56-2.65(2H,m),2.97-3.18(2H,m),4.05-4.23(1H,m),4.05-4.23(1H,m),4.85(1H, br s), 5.08(2H,m).
[0050] Step three:
[0051] Compound 3 (8.0 g, 0.044 mol) was dissolved in dichloromethane (50 mL) and water (50 mL). Then, add RuCl to this reaction solution 3 (0.32g), NaIO 4 (14.1 g, 0.066 mol). After stirring at room temperature for 1 hour, the aqueous pha...
Embodiment 3
[0056] step one:
[0057] Same as Step 1 in Example 1.
[0058] Step two:
[0059] N,N-diisopropylethylamine (762.5g, 5.9mol), TMSN 3 (541.4g, 4.7mol) and compound 2 (440g, 3.9mol) were dissolved in tert-butanol (8L) at a reflux temperature of 83°C, and then refluxed for 12 hours. The reaction was quenched with water (2 L), and after the solvent was concentrated, ethyl acetate (7.8 L) was added to the remaining solution. The organic phase was washed with water, washed with saturated brine, dried, and spin-dried to obtain a white solid, compound 3 (534.8 g), with a yield of 75%. Proton NMR spectrum (400MHz, CDCl 3 )δ:1.36(9H,s),2.56-2.65(2H,m),2.97-3.18(2H,m),4.05-4.23(1H,m),4.05-4.23(1H,m),4.85(1H, br s), 5.08(2H,m).
[0060] Step three:
[0061] Compound 3 (8.0 g, 0.044 mol) was dissolved in DMF (80 mL). Then, add OsO to this reaction solution 4 (0.11 g) and tert-butanol peroxy (26.1 g, 0.176 mol). After stirring at room temperature for 3 hours, ethyl acetate (100 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com