Preparation method of beta-homoglutamic acid
A high-glutamic acid, high-yield technology, applied in the preparation of carbamic acid derivatives, organic compounds, organic chemical methods, etc., can solve the problems of cumbersome preparation, high cost, and unsuitable scale-up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
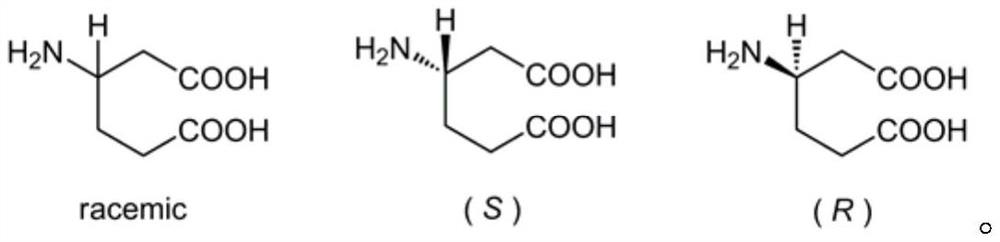
[0012] (1) Preparation method of racemic β-homoglutamic acid
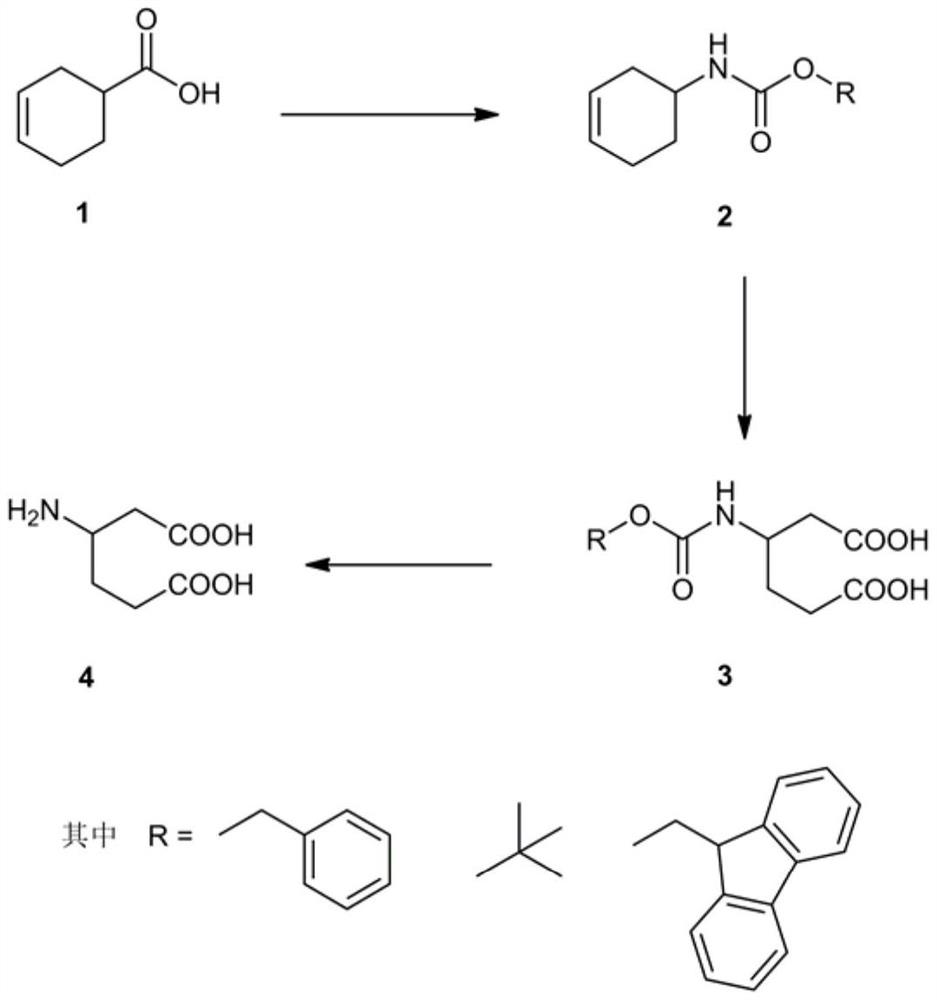
[0013] The synthesis of racemic β-homoglutamic acid in the present invention adopts racemic 3-cyclohexene carboxylic acid as raw material, utilizes diphenylphosphoryl azide to synthesize acyl azide, obtains isocyanate through Curtius rearrangement, and then adds benzyl alcohol Or tert-butanol or fluorene methanol one-pot reaction to generate benzyl cyclohexenyl carbamate or tert-butyl cyclohexenyl carbamate or fluorenylmethyl cyclohexenyl carbamate, and then use potassium permanganate to carry out olefin The diacid intermediate is obtained by oxidative cleavage, and the racemic β-homoglutamic acid can be obtained by removing the protecting group Cbz or Boc or Fmoc at last.
[0014] The solvents used in the Curtius rearrangement reaction are toluene and xylene, and the reaction temperature is 100° C. to 140° C.
[0015] (2) Preparation method of optically pure β-homoglutamic acid
[0016] In the present invention,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com