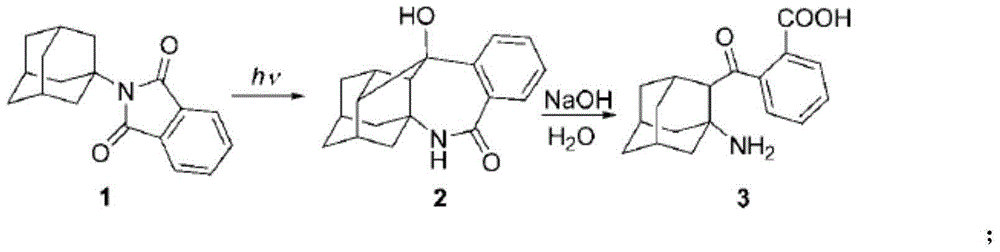
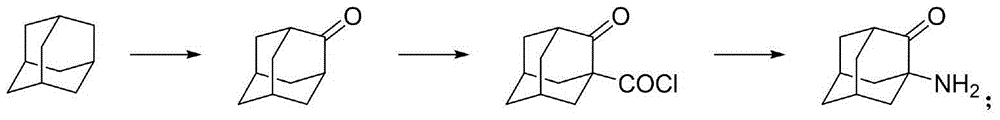
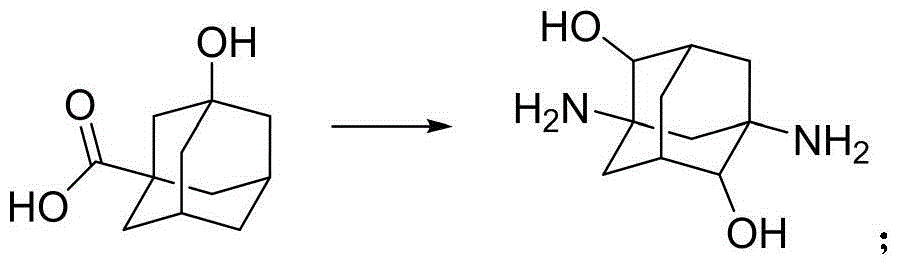Adamantane amine derivative as well as preparation method and application of derivative
A technology for amantadine and adamantanecarboxamide, applied in the field of amantadine derivatives and their preparation, can solve the problems of complex preparation method steps, single type of groups and the like, and achieve prevention and treatment of biological activity and cell death rate. The effect of reducing and protecting nerve cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the synthesis of compound A-1
[0042] S1 Add 15 mL of thionyl chloride to 3,5-dimethyladamantane-1-carboxylic acid (28.8 mmol, 6 g), drop 3 drops of DMF into the solution, reflux at 80°C for 2 hours, and spin the reaction solution to dryness Remove excess thionyl chloride. The residue was dissolved in 50 mL of anhydrous toluene, 2,3,5,6-tetrafluoro-4-(trifluoromethyl)aniline (14 mmol, 3.4 g) was added, and the mixture was refluxed at 120° C. for 12 hours. Spin to dry the solvent of the reaction solution, and recrystallize from petroleum ether to obtain 3,5-dimethyl-N-(2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl)-1-adamantane Formamide, white solid, yield 64% (based on aniline).
[0043]
[0044]3,5-Dimethyl-N-(2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl)-1-adamantanecarboxamide
[0045] S2: 3,5-dimethyl-N-(2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl)-1-adamantanecarboxamide (0.1mmol), Pd( TFA) 2 (0.01mmol), m-chloroiodobenzene (0.5mmol), PPh...
Embodiment 2
[0054] Embodiment 2: the synthesis of compound A-2
[0055] S1: Add 1-adamantanecarboxylic acid (40.8mmol, 7.4g) to 16ml of thionyl chloride solution, then drop 3 drops of DMF, reflux at 80°C for 2 hours, and spin the reaction solution to dry to remove excess dichloride. Thionyl chloride. The residue was dissolved in 50 mL of anhydrous toluene, 2,3,5,6-tetrafluoro-4-(trifluoromethyl)aniline (10 mmol, 2.4 g) was added, refluxed at 120 ° C for 12 hours, and the solvent of the reaction solution was spin-dried. Recrystallization from petroleum ether gave N-(2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl)-1-adamantanecarboxamide, white solid, yield 73% (based on aniline) .
[0056]
[0057] N-(2,3,5,6-Tetrafluoro-4-(trifluoromethyl)phenyl)-1-adamantanecarboxamide
[0058] S2. Weigh the obtained in step S1. N-(2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl)-1-adamantanecarboxamide (0.1mmol), Pd(TFA) 2 (0.01mmol), p-iodoanisole (0.5mmol), PPh 3 (0.05mmol), CsF (0.3mmol) and n-h...
Embodiment 3
[0066] Embodiment 3: the synthesis of compound A-3
[0067] The preparation method and steps were the same as in Example 2, except that the intermediate chloroiodobenzene (0.5 mmol) in the S2 step was replaced with iodoethane (0.5 mmol) to obtain the final product A-3, a white solid, with a yield of 55%.
[0068]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com