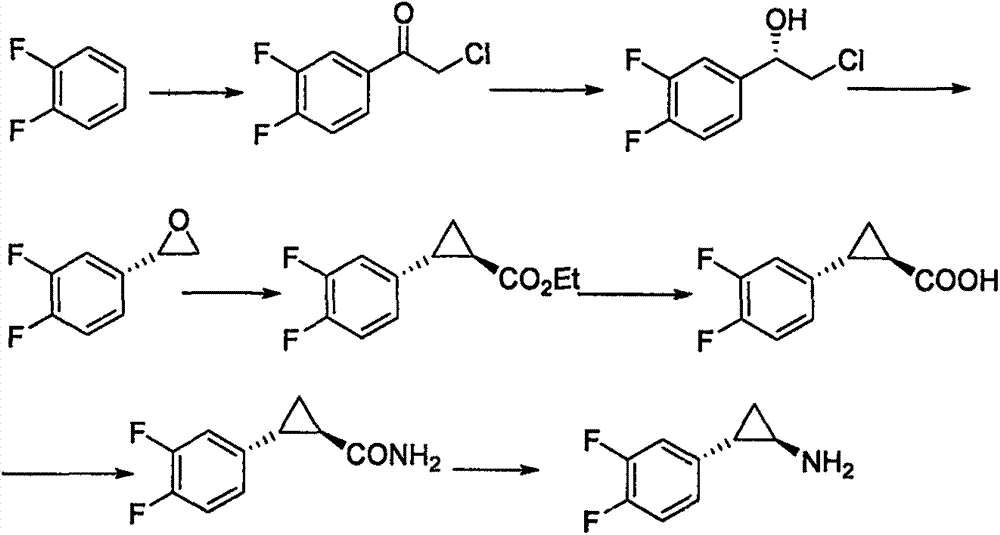
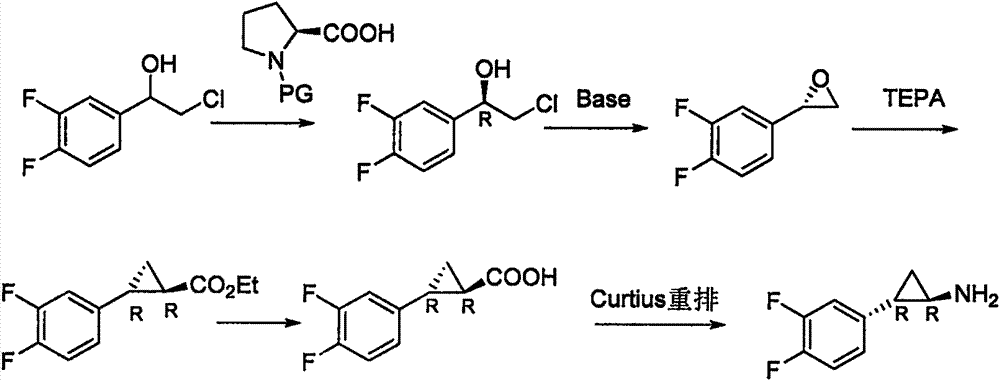
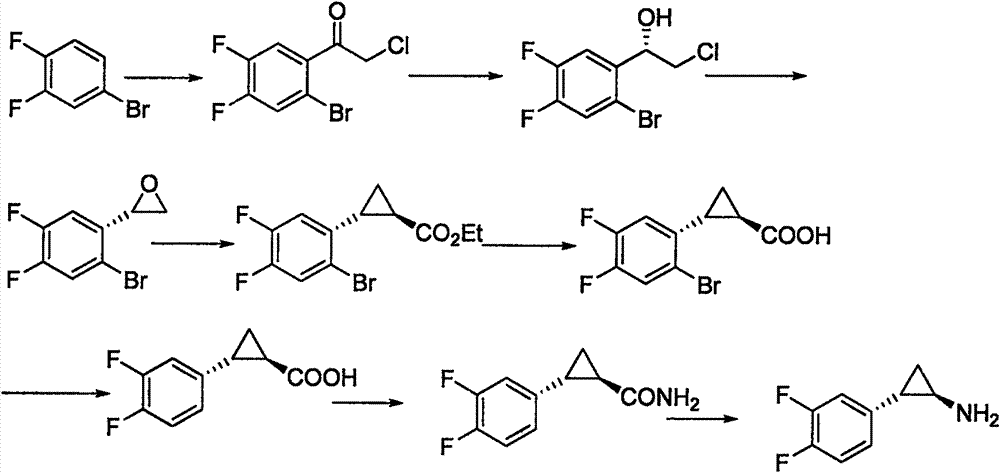
Preparation method for ticagrelor intermediate and mandelate thereof
A technology for ticagrelor and intermediates, which is applied in the field of synthesis of ticagrelor intermediates-2-cyclopropylamine and its mandelic acid salts, can solve the problems of high production cost, poor optical purity, cumbersome operation, etc., and achieve Less by-products, less pollution, better optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: the cultivation of carbonyl reductase thalline
[0052] Seed culture:
[0053] Medium formula: peptone 1.0%, yeast extract powder 0.5%, sodium chloride 0.5%, pH 7.0 before disinfection, volume before disinfection 100mL (1000mL shake flask), cool after sterilization, add 100uL to the seed bottle under sterile condition The concentration is 10mg / mL ampicillin and 0.1mL glycerol tube bacteria solution (Escherichia coli, E.Coli BL21), cultured on a shaker at 37°C with a rotation speed of 200rpm, and the culture time is 18-20 hours.
[0054] Fermentation culture:
[0055]Medium formula: peptone 2.0%, yeast extract powder 1.0%, sodium chloride 0.5%, foam enemy (defoamer) 0.02%, glycerol 0.2%. The pH before disinfection is 7.5, and the volume before disinfection is 5L (10L fermenter); after the medium is sterilized, it is inoculated into the cultivated seed culture solution for fermentation by flame inoculation, and the culture conditions are fermentation tempe...
Embodiment 2
[0056] Example 2: Preparation of 2-chloro-1-S-(3,4-difluorophenyl)ethanol (II)
[0057] Add 40g of 2-chloro-1-(3,4-difluorophenyl)ethanone, 200ml of water and 100ml of isopropanol in a mixed solvent in a stirred 500ml four-neck flask, start stirring, and add Example 1 20 g of the bacterium containing carbonyl reductase obtained in , the temperature was raised to 30 ° C to start the reaction, after 3 h, samples were taken every 0.5 h and the residue of 2-chloro-1-(3,4-difluorophenyl)ethanone was detected by HPLC. After the reaction was completed, the cells were removed by filtration, the filtrate was extracted with toluene, the toluene phase was dried with anhydrous magnesium sulfate, filtered, and concentrated to obtain 39.5 g of yellow oily compound (II), with a yield of 98.8%, HPLC purity of 98.5%, and ee value of 99.9%.
Embodiment 3
[0058] Example 3: Preparation of 2-chloro-1-S-(3,4-difluorophenyl)ethanol (II)
[0059] In a stirred 500ml four-neck flask, sequentially add 40g of 2-chloro-1-(3,4-difluorophenyl)ethanone, 200ml of water and 40ml of tetrahydrofuran in a mixed solvent, start stirring, and add the 12g of bacteria containing carbonyl reductase, the temperature was raised to 40°C to start the reaction, after 3h, samples were taken every 0.5h and the residue of 2-chloro-1-(3,4-difluorophenyl)ethanone was detected by HPLC. The bacteria were removed by filtration, the filtrate was extracted with EA, the EA phase was dried with anhydrous magnesium sulfate, filtered, and concentrated to obtain 39.1 g of yellow oily compound (II), with a yield of 96.7%, an HPLC purity of 98.6%, and an ee value of 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com