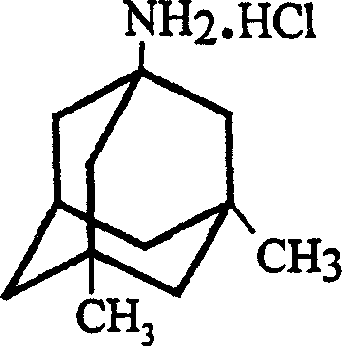
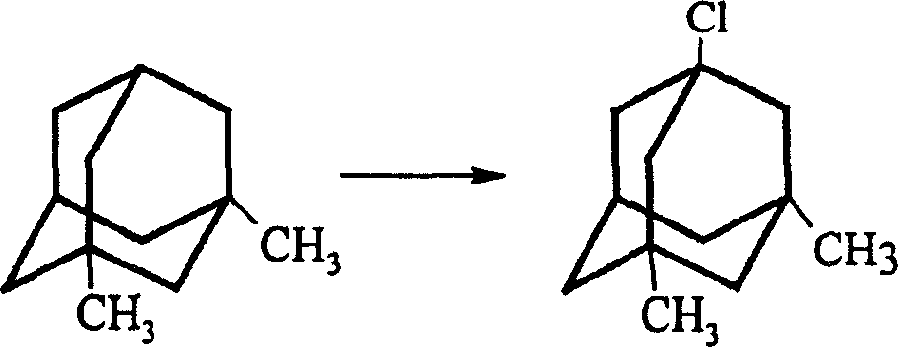
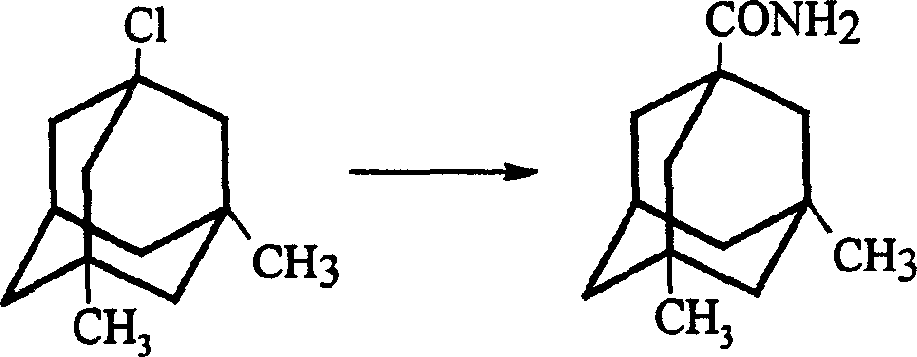Method for preparing memantine hydrochloride
A technology of memantine hydrochloride and dimethyladamantane, which is applied in the field of senile dementia treatment drugs, can solve the problems of easy to cause combustion and explosion accidents, high ventilation performance requirements, easy environmental pollution, etc. The effect of recycling, low cost and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of 1-chloro-3,5-dimethyladamantane:
Embodiment 2
[0027] Synthesis of 1-Acetylamino-3,5-Dimethyladamantane:
[0028] Add 147.3g of 1-chloro-3,5-dimethyladamantane and 380.5g of acetamide obtained in the previous step (Example 1) into a 1000ml round-bottomed flask. The heating temperature is controlled at 20~120℃, and the reaction is refluxed under stirring. After 3 hours, pour into 1000ml of water, stand still overnight, precipitate a solid, filter with suction, wash with water, and dry to obtain 151.3g of crude product, which is recrystallized with petroleum ether to obtain 145.9g of white needle-like crystals. The two-step yield totaled 89.0%. The melting point is 113.5-114.5°C.
Embodiment 3
[0030] Synthesis of 1-amino-3,5-dimethylamantadine hydrochloride:
[0031] Add 12.2g sodium hydroxide and 350ml glycerol to a 1000ml round bottom flask, heat to 100~110℃, stir and reflux until the sodium hydroxide is completely dissolved, the solution gradually turns black, add 145.9g after the sodium hydroxide is dissolved 1- Acetylamino-3,5-dimethyladamantane is heated to 150-160°C under stirring and reflux, and the reaction is continued at this temperature for 6 hours. After the reaction was cooled to room temperature, it was poured into ice water, extracted three times with ethyl acetate, the organic layers were combined, dried over anhydrous sodium sulfate, filtered, filtered with suction, and concentrated to obtain a yellow oil as 1-amino-3,5- Crude dimethylamantadine. Dry hydrogen chloride gas was passed through under stirring, and solid was precipitated. The solid was filtered by suction. The obtained solid was recrystallized with ethanol-ethyl acetate (3:5v / v) mixed solve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com